White matter injury across neurodegenerative disease
- PMID: 38052682
- PMCID: PMC10842057
- DOI: 10.1016/j.tins.2023.11.003
White matter injury across neurodegenerative disease
Abstract
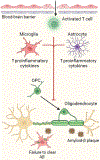
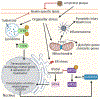
Oligodendrocytes (OLs), the myelin-generating cells of the central nervous system (CNS), are active players in shaping neuronal circuitry and function. It has become increasingly apparent that injury to cells within the OL lineage plays a central role in neurodegeneration. In this review, we focus primarily on three degenerative disorders in which white matter loss is well documented: Alzheimer's disease (AD), Parkinson's disease (PD), and amyotrophic lateral sclerosis (ALS). We discuss clinical data implicating white matter injury as a key feature of these disorders, as well as shared and divergent phenotypes between them. We examine the cellular and molecular mechanisms underlying the alterations to OLs, including chronic neuroinflammation, aggregation of proteins, lipid dysregulation, and organellar stress. Last, we highlight prospects for therapeutic intervention targeting the OL lineage to restore function.
Keywords: Alzheimer’s disease; Parkinson’s disease; amyotrophic lateral sclerosis; lipid dysregulation; neuroinflammation; organellar stress.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

References
-
- Kaes T. (1907) Die Grosshirnrinde des Menschen in ihren Massen und in ihrem Fasergehalt: ein gehirnanatomischer Atlas, mit erläuterndem Text, Fischer
-
- de Faria O Jr et al. (2021) Periods of synchronized myelin changes shape brain function and plasticity. Nat. Neurosci 24, 1508–1521 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

