Dental impact of anti-fibroblast growth factor 23 therapy in X-linked hypophosphatemia
- PMID: 38052774
- PMCID: PMC10697996
- DOI: 10.1038/s41368-023-00259-8
Dental impact of anti-fibroblast growth factor 23 therapy in X-linked hypophosphatemia
Abstract
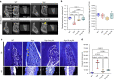
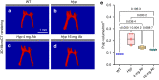
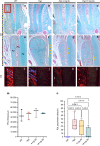
Elevated fibroblast growth factor 23 (FGF23) in X-linked hypophosphatemia (XLH) results in rickets and phosphate wasting, manifesting by severe bone and dental abnormalities. Burosumab, a FGF23-neutralizing antibody, an alternative to conventional treatment (phosphorus and active vitamin D analogs), showed significant improvement in the long bone phenotype. Here, we examined whether FGF23 antibody (FGF23-mAb) also improved the dentoalveolar features associated with XLH. Four-week-old male Hyp mice were injected weekly with 4 or 16 mg·kg-1 of FGF23-mAb for 2 months and compared to wild-type (WT) and vehicle (PBS) treated Hyp mice (n = 3-7 mice). Micro-CT analyses showed that both doses of FGF23-mAb restored dentin/cementum volume and corrected the enlarged pulp volume in Hyp mice, the higher concentration resulting in a rescue similar to WT levels. FGF23-mAb treatment also improved alveolar bone volume fraction and mineral density compared to vehicle-treated ones. Histology revealed improved mineralization of the dentoalveolar tissues, with a decreased amount of osteoid, predentin and cementoid. Better periodontal ligament attachment was also observed, evidenced by restoration of the acellular cementum. These preclinical data were consistent with the retrospective analysis of two patients with XLH showing that burosumab treatment improved oral features. Taken together, our data show that the dentoalveolar tissues are greatly improved by FGF23-mAb treatment, heralding its benefit in clinics for dental abnormalities.
© 2023. The Author(s).
Conflict of interest statement
K.N., A.H. and T.S. are industry scientists at Kyowa Kirin. A.L. reports a research grant from Kyowa Kirin, honoraria, and consulting fees from Pfizer, Novonordisk, Merck Serono and Sandoz, unrelated to this work. M.B.D. reports a research grant and honoraria from Kyowa Kirin. C.C. reports a research collaborative grant from Kyowa Kirin for URP2496 related to this work, and consulting fees from Novonordisk for URP2496 unrelated to this work. The other authors report no conflicts of interest.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
- SPF202209015771/Fondation pour la Recherche Médicale (Foundation for Medical Research in France)
- SPF202209015771/Fondation pour la Recherche Médicale (Foundation for Medical Research in France)
- 18-CE14-0018-01/Agence Nationale de la Recherche (French National Research Agency)
- ANR-11-INBS-0006/Agence Nationale de la Recherche (French National Research Agency)
LinkOut - more resources
Full Text Sources
Medical

