Metformin ameliorates valve interstitial cell calcification by promoting autophagic flux
- PMID: 38052777
- PMCID: PMC10698150
- DOI: 10.1038/s41598-023-47774-6
Metformin ameliorates valve interstitial cell calcification by promoting autophagic flux
Abstract
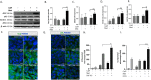
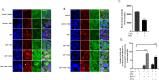
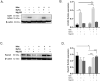
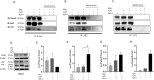
Calcific aortic valve disease (CAVD) is the most common heart disease of the developed world. It has previously been established that metformin administration reduces arterial calcification via autophagy; however, whether metformin directly regulates CAVD has yet to be elucidated. In the present study we investigated whether metformin alleviates valvular calcification through the autophagy-mediated recycling of Runx2. Calcification was reduced in rat valve interstitial cells (RVICs) by metformin treatment (0.5-1.5 mM) (P < 0.01), with a marked decrease in Runx2 protein expression compared to control cells (P < 0.05). Additionally, upregulated expression of Atg3 and Atg7 (key proteins required for autophagosome formation), was observed following metformin treatment (1 mM). Blocking autophagic flux using Bafilomycin-A1 revealed colocalisation of Runx2 with LC3 puncta in metformin treated RVICs (P < 0.001). Comparable Runx2 accumulation was seen in LC3 positive autolysosomes present within cells that had been treated with both metformin and hydroxychloroquine in combination (P < 0.001). Mechanistic studies employing three-way co-immunoprecipitation with Runx2, p62 and LC3 suggested that Runx2 binds to LC3-II upon metformin treatment in VICs. Together these studies suggest that the utilisation of metformin may represent a novel strategy for the treatment of CAVD.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Metformin protects against vascular calcification through the selective degradation of Runx2 by the p62 autophagy receptor.J Cell Physiol. 2022 Nov;237(11):4303-4316. doi: 10.1002/jcp.30887. Epub 2022 Sep 27. J Cell Physiol. 2022. PMID: 36166694
-
Metformin alleviates the calcification of aortic valve interstitial cells through activating the PI3K/AKT pathway in an AMPK dependent way.Mol Med. 2021 Dec 11;27(1):156. doi: 10.1186/s10020-021-00416-x. Mol Med. 2021. PMID: 34895136 Free PMC article.
-
Long noncoding TSI attenuates aortic valve calcification by suppressing TGF-β1-induced osteoblastic differentiation of valve interstitial cells.Metabolism. 2023 Jan;138:155337. doi: 10.1016/j.metabol.2022.155337. Epub 2022 Oct 21. Metabolism. 2023. PMID: 36273649
-
Transforming growth factor-β1 promotes fibrosis but attenuates calcification of valvular tissue applied as a three-dimensional calcific aortic valve disease model.Am J Physiol Heart Circ Physiol. 2020 Nov 1;319(5):H1123-H1141. doi: 10.1152/ajpheart.00651.2019. Epub 2020 Sep 28. Am J Physiol Heart Circ Physiol. 2020. PMID: 32986963
-
Oxidative stress and valvular endothelial cells in aortic valve calcification.Biomed Pharmacother. 2023 Jul;163:114775. doi: 10.1016/j.biopha.2023.114775. Epub 2023 Apr 26. Biomed Pharmacother. 2023. PMID: 37116353 Review.
Cited by
-
Diabetes and calcific aortic valve disease: implications of glucose-lowering medication as potential therapy.Front Pharmacol. 2025 Apr 28;16:1583267. doi: 10.3389/fphar.2025.1583267. eCollection 2025. Front Pharmacol. 2025. PMID: 40356984 Free PMC article. Review.
-
The Role of NOTCH Pathway Genes in the Inherited Susceptibility to Aortic Stenosis.J Cardiovasc Dev Dis. 2024 Jul 17;11(7):226. doi: 10.3390/jcdd11070226. J Cardiovasc Dev Dis. 2024. PMID: 39057646 Free PMC article.
-
Nutrient restriction protects against valve interstitial cell calcification by upregulating ubiquitin mediated proteolysis.Front Cardiovasc Med. 2025 Jul 21;12:1586775. doi: 10.3389/fcvm.2025.1586775. eCollection 2025. Front Cardiovasc Med. 2025. PMID: 40761233 Free PMC article.
References
-
- Rajamannan NM, et al. Calcific aortic valve disease: Not simply a degenerative process: A review and agenda for research from the national heart and lung and blood institute aortic stenosis working Group. Executive summary: Calcific aortic valve disease-2011 update. Circulation. 2011;124:1783–1791. doi: 10.1161/CIRCULATIONAHA.110.006767. - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

