Choroidal and retinal thinning in chronic kidney disease independently associate with eGFR decline and are modifiable with treatment
- PMID: 38052813
- PMCID: PMC10697963
- DOI: 10.1038/s41467-023-43125-1
Choroidal and retinal thinning in chronic kidney disease independently associate with eGFR decline and are modifiable with treatment
Abstract
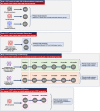
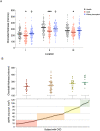
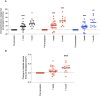
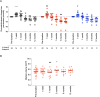
In patients with chronic kidney disease (CKD), there is an unmet need for novel biomarkers that reliably track kidney injury, demonstrate treatment-response, and predict outcomes. Here, we investigate the potential of retinal optical coherence tomography (OCT) to achieve these ends in a series of prospective studies of patients with pre-dialysis CKD (including those with a kidney transplant), patients with kidney failure undergoing kidney transplantation, living kidney donors, and healthy volunteers. Compared to health, we observe similar retinal thinning and reduced macular volume in patients with CKD and in those with a kidney transplant. However, the choroidal thinning observed in CKD is not seen in patients with a kidney transplant whose choroids resemble those of healthy volunteers. In CKD, the degree of choroidal thinning relates to falling eGFR and extent of kidney scarring. Following kidney transplantation, choroidal thickness increases rapidly (~10%) and is maintained over 1-year, whereas gradual choroidal thinning is seen during the 12 months following kidney donation. In patients with CKD, retinal and choroidal thickness independently associate with eGFR decline over 2 years. These observations highlight the potential for retinal OCT to act as a non-invasive monitoring and prognostic biomarker of kidney injury.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

