The Cantabria Cohort, a protocol for a population-based cohort in northern Spain
- PMID: 38053113
- PMCID: PMC10698930
- DOI: 10.1186/s12889-023-17318-8
The Cantabria Cohort, a protocol for a population-based cohort in northern Spain
Abstract
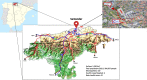
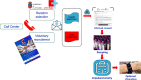
Cantabria Cohort stems from a research and action initiative lead by researchers from Valdecilla Research Institute (IDIVAL), Marqués de Valdecilla University Hospital and University of Cantabria, supported by the regional Goverment. Its aim is to identify and follow up a cohort that would provide information to improve the understanding of the etiology and prognosis of different acute and chronic diseases. The Cantabria Cohort will recruit between 40,000-50,000 residents aged 40-69 years at baseline, representing 10-20% of the target population. Currently, more than 30,000 volunteers have been enrolled. All participants will be invited for a re-assessment every three years, while the overall duration is planned for twenty years. The repeated collection of biomaterials combined with broad information from participant questionnaires, medical examinations, actual health system records and other secondary public data sources is a major strength of its design, which will make it possible to address biological pathways of disease development, identify new factors involved in health and disease, design new strategies for disease prevention, and advance precision medicine. It is conceived to allow access to a large number of researchers worldwide to boost collaboration and medical research.
Keywords: Big data; Biobank; Lifestyle; Longitudinal study; Population-based cohort; Precision medicine; Socio-economic factors; Spain.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Nonetheless, they stated that: M.A.P. received a fellowship from Hoffmann-La Roche; J.V.L. reports grants from AbbVie, Gilead Sciences, MSD, and Roche Diagnostics; consulting fees from Novo-Vax; payment or honoraria for lectures, presentations, speakers’ bureaus, and educational events from AbbVie, Gilead, Sciences, Intercept, and Janssen; M.L.H. reports grants from Werfen and ThermoFisher; consulting fees from Werfen and ThermoFisher; payment or honoraria for lectures, presentations, speakers’ bureaus, and educational events from Werfen, ThermoFisher, Sanofi, GSK, Astra Zeneca, UCB Pharma, Astellas, and Takeda. The rest of the authors have no competing interest to declare.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

