Dengue and Zika RNA-RNA interactomes reveal pro- and anti-viral RNA in human cells
- PMID: 38053173
- PMCID: PMC10696742
- DOI: 10.1186/s13059-023-03110-9
Dengue and Zika RNA-RNA interactomes reveal pro- and anti-viral RNA in human cells
Abstract
Background: Identifying host factors is key to understanding RNA virus pathogenicity. Besides proteins, RNAs can interact with virus genomes to impact replication.
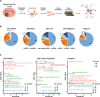
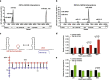
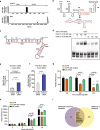
Results: Here, we use proximity ligation sequencing to identify virus-host RNA interactions for four strains of Zika virus (ZIKV) and one strain of dengue virus (DENV-1) in human cells. We find hundreds of coding and non-coding RNAs that bind to DENV and ZIKV viruses. Host RNAs tend to bind to single-stranded regions along the virus genomes according to hybridization energetics. Compared to SARS-CoV-2 interactors, ZIKV-interacting host RNAs tend to be downregulated upon virus infection. Knockdown of several short non-coding RNAs, including miR19a-3p, and 7SK RNA results in a decrease in viral replication, suggesting that they act as virus-permissive factors. In addition, the 3'UTR of DYNLT1 mRNA acts as a virus-restrictive factor by binding to the conserved dumbbell region on DENV and ZIKV 3'UTR to decrease virus replication. We also identify a conserved set of host RNAs that interacts with DENV, ZIKV, and SARS-CoV-2, suggesting that these RNAs are broadly important for RNA virus infection.
Conclusions: This study demonstrates that host RNAs can impact virus replication in permissive and restrictive ways, expanding our understanding of host factors and RNA-based gene regulation during viral pathogenesis.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

