Prognostic Biomarkers in Kidney Transplantation: A Systematic Review and Critical Appraisal
- PMID: 38053242
- PMCID: PMC10843205
- DOI: 10.1681/ASN.0000000000000260
Prognostic Biomarkers in Kidney Transplantation: A Systematic Review and Critical Appraisal
Abstract
Significance statement: Why are there so few biomarkers accepted by health authorities and implemented in clinical practice, despite the high and growing number of biomaker studies in medical research ? In this meta-epidemiological study, including 804 studies that were critically appraised by expert reviewers, the authors have identified all prognostic kidney transplant biomarkers and showed overall suboptimal study designs, methods, results, interpretation, reproducible research standards, and transparency. The authors also demonstrated for the first time that the limited number of studies challenged the added value of their candidate biomarkers against standard-of-care routine patient monitoring parameters. Most biomarker studies tended to be single-center, retrospective studies with a small number of patients and clinical events. Less than 5% of the studies performed an external validation. The authors also showed the poor transparency reporting and identified a data beautification phenomenon. These findings suggest that there is much wasted research effort in transplant biomarker medical research and highlight the need to produce more rigorous studies so that more biomarkers may be validated and successfully implemented in clinical practice.
Background: Despite the increasing number of biomarker studies published in the transplant literature over the past 20 years, demonstrations of their clinical benefit and their implementation in routine clinical practice are lacking. We hypothesized that suboptimal design, data, methodology, and reporting might contribute to this phenomenon.
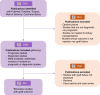
Methods: We formed a consortium of experts in systematic reviews, nephrologists, methodologists, and epidemiologists. A systematic literature search was performed in PubMed, Embase, Scopus, Web of Science, and Cochrane Library between January 1, 2005, and November 12, 2022 (PROSPERO ID: CRD42020154747). All English language, original studies investigating the association between a biomarker and kidney allograft outcome were included. The final set of publications was assessed by expert reviewers. After data collection, two independent reviewers randomly evaluated the inconsistencies for 30% of the references for each reviewer. If more than 5% of inconsistencies were observed for one given reviewer, a re-evaluation was conducted for all the references of the reviewer. The biomarkers were categorized according to their type and the biological milieu from which they were measured. The study characteristics related to the design, methods, results, and their interpretation were assessed, as well as reproducible research practices and transparency indicators.
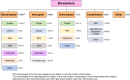
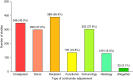
Results: A total of 7372 publications were screened and 804 studies met the inclusion criteria. A total of 1143 biomarkers were assessed among the included studies from blood ( n =821, 71.8%), intragraft ( n =169, 14.8%), or urine ( n =81, 7.1%) compartments. The number of studies significantly increased, with a median, yearly number of 31.5 studies (interquartile range [IQR], 23.8-35.5) between 2005 and 2012 and 57.5 (IQR, 53.3-59.8) between 2013 and 2022 ( P < 0.001). A total of 655 studies (81.5%) were retrospective, while 595 (74.0%) used data from a single center. The median number of patients included was 232 (IQR, 96-629) with a median follow-up post-transplant of 4.8 years (IQR, 3.0-6.2). Only 4.7% of studies were externally validated. A total of 346 studies (43.0%) did not adjust their biomarker for key prognostic factors, while only 3.1% of studies adjusted the biomarker for standard-of-care patient monitoring factors. Data sharing, code sharing, and registration occurred in 8.8%, 1.1%, and 4.6% of studies, respectively. A total of 158 studies (20.0%) emphasized the clinical relevance of the biomarker, despite the reported nonsignificant association of the biomarker with the outcome measure. A total of 288 studies assessed rejection as an outcome. We showed that these rejection studies shared the same characteristics as other studies.
Conclusions: Biomarker studies in kidney transplantation lack validation, rigorous design and methodology, accurate interpretation, and transparency. Higher standards are needed in biomarker research to prove the clinical utility and support clinical use.
Copyright © 2023 by the American Society of Nephrology.
Conflict of interest statement
O. Aubert reports Honoraria: CareDX and Novartis. É. Bailly reports Ownership Interest: Gilead, Johnson & Johnson, Merck, Novartis, and Pfizer. O. Bestard reports Patents or Royalties: Oxford Immunotec. A. Del Bello reports Speakers Bureau: Hansa Pharma and Neovii. J.J. Friedewald reports Consultancy: egenesis, Eurofins—Transplant Genomics, Inc., and Veloxis; Research Funding: CSL Behring, Eurofins—Viracor, Inc., Hansa BioPharma, NIH, Regeneron, and Veloxis; Honoraria: Sanofi and Up to Date; Patents or Royalties: Northwestern University/Scripps Research Institute; and Speakers Bureau: Sanofi. C. Lefaucheur reports Consultancy: Hansa Biopharm; Research Funding: EU Train/H2020 and KTD-Innov/ANR France; Advisory or Leadership Role: Chiesi; and Speakers Bureau: Astellas and Hansa Biopharma for ESOT Symposium 2023. M. Naesens reports Consultancy: Agomab, Aiosyn, Argenx, and Hansa; Research Funding: CareDx; Honoraria: Argenx and Hansa; and Patents or Royalties: inventor of two patents related to the FWO-SBO application; EP19152365.3: mRNA-based biomarkers for antibody-mediated transplant rejection. This biomarker was licensed in September 2020 to CareDx, a precision medicine solutions company focused on solutions for transplant patients; PCT/EP2018/097044: Biomarkers for typing allograft recipients (patent application submitted December 2018). L. Riella reports Consultancy: Caredx; Research Funding: Brystol-Meyers Squibb, Caredx, Natera, Sanofi, and Visterra; Honoraria: Caredx; and Advisory or Leadership Role: CareDx. C. Wang reports Research Funding: Astellas Pharma Inc. All remaining authors have nothing to disclose.
Figures

References
-
- WHO International Programme on Chemical Safety Biomarkers in Risk Assessment: Validity and Validation. 2001. http://www.inchem.org/documents/ehc/ehc/ehc222.htm
Publication types
MeSH terms
Substances
Grants and funding
- ANR-17-RHUS-0010/French government financial support managed by the National Research Agency (ANR) under the program “Investissements d’avenir†KTD-Innov
- 754995/European Union’s Horizon 2020 research and innovation program EU-TRAIN
- ANR-17-RHUS-0010/French government financial support managed by the National Research Agency (ANR) under the program "Investissements d'avenir" KTD-Innov
- 754995/European Union's Horizon 2020 research and innovation program EU-TRAIN
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

