First shotgun metagenomics study of Juan de Fuca deep-sea sediments reveals distinct microbial communities above, within, between, and below sulfate methane transition zones
- PMID: 38053553
- PMCID: PMC10694467
- DOI: 10.3389/fmicb.2023.1241810
First shotgun metagenomics study of Juan de Fuca deep-sea sediments reveals distinct microbial communities above, within, between, and below sulfate methane transition zones
Abstract
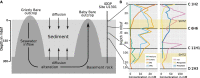
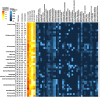
The marine deep subsurface is home to a vast microbial ecosystem, affecting biogeochemical cycles on a global scale. One of the better-studied deep biospheres is the Juan de Fuca (JdF) Ridge, where hydrothermal fluid introduces oxidants into the sediment from below, resulting in two sulfate methane transition zones (SMTZs). In this study, we present the first shotgun metagenomics study of unamplified DNA from sediment samples from different depths in this stratified environment. Bioinformatic analyses showed a shift from a heterotrophic, Chloroflexota-dominated community above the upper SMTZ to a chemolithoautotrophic Proteobacteria-dominated community below the secondary SMTZ. The reintroduction of sulfate likely enables respiration and boosts active cells that oxidize acetate, iron, and complex carbohydrates to degrade dead biomass in this low-abundance, low-diversity environment. In addition, analyses showed many proteins of unknown function as well as novel metagenome-assembled genomes (MAGs). The study provides new insights into microbial communities in this habitat, enabled by an improved DNA extraction protocol that allows a less biased view of taxonomic composition and metabolic activities, as well as uncovering novel taxa. Our approach presents the first successful attempt at unamplified shotgun sequencing samples from beyond 50 meters below the seafloor and opens new ways for capturing the true diversity and functional potential of deep-sea sediments.
Keywords: archaea; bacteria; deep biosphere; hydrothermal fluid; marine subsurface; metagenome assembled genomes; optimized DNA extraction.
Copyright © 2023 Metze, Vollmers, Lenk and Kaster.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Alneberg J, Bjarnason BS, Bruijn I, Schirmer M, Quick J, Ijaz UZ, et al. . (2013). CONCOCT: Clustering cONtigs on COverage and ComposiTion. Available at: https://arxiv.org/pdf/1312.4038 (Accessed August, 2020).
-
- Andrews S. (2010). FastQC: FastQC a quality control tool for high throughput sequence data. Available at: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ [Accessed August 28, 2020].
-
- BamM: Working with the BAM (2016). Available at: http://ecogenomics.github.io/BamM/ [Accessed April 9, 2020].
LinkOut - more resources
Full Text Sources

