Revolutionizing biomedicine: advancements, applications, and prospects of nanocomposite macromolecular carbohydrate-based hydrogel biomaterials: a review
- PMID: 38053691
- PMCID: PMC10694639
- DOI: 10.1039/d3ra07391b
Revolutionizing biomedicine: advancements, applications, and prospects of nanocomposite macromolecular carbohydrate-based hydrogel biomaterials: a review
Abstract
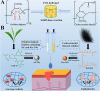
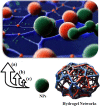
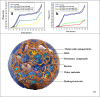
Nanocomposite hydrogel biomaterials represent an exciting Frontier in biomedicine, offering solutions to longstanding challenges. These hydrogels are derived from various biopolymers, including fibrin, silk fibroin, collagen, keratin, gelatin, chitosan, hyaluronic acid, alginate, carrageenan, and cellulose. While these biopolymers possess inherent biocompatibility and renewability, they often suffer from poor mechanical properties and rapid degradation. Researchers have integrated biopolymers such as cellulose, starch, and chitosan into hydrogel matrices to overcome these limitations, resulting in nanocomposite hydrogels. These innovative materials exhibit enhanced mechanical strength, improved biocompatibility, and the ability to finely tune drug release profiles. The marriage of nanotechnology and hydrogel chemistry empowers precise control over these materials' physical and chemical properties, making them ideal for tissue engineering, drug delivery, wound healing, and biosensing applications. Recent advancements in the design, fabrication, and characterization of biopolymer-based nanocomposite hydrogels have showcased their potential to transform biomedicine. Researchers are employing strategic approaches for integrating biopolymer nanoparticles, exploring how nanoparticle properties impact hydrogel performance, and utilizing various characterization techniques to evaluate structure and functionality. Moreover, the diverse biomedical applications of these nanocomposite hydrogels hold promise for improving patient outcomes and addressing unmet clinical needs.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures







References
-
- Chen W. Zhang C. Peng S. Lin Y. Ye Z. Adv. Ther. 2023:2300128.
-
- Gao F. Jiao C. Yu B. Cong H. Shen Y. Mater. Chem. Front. 2021;5:4912–4936.
-
- Castrejón-Comas V. Alemán C. Pérez-Madrigal M. M. Biomater. Sci. 2023;11:2266–2276. - PubMed
-
- Anwer A. H. Ahtesham A. Shoeb M. Mashkoor F. Ansari M. Z. Zhu S. Jeong C. Adv. Colloid Interface Sci. 2023:102955. - PubMed
-
- Zhang Z. Hao Z. Xian C. Zhang J. Wu J. Chem. Eng. J. 2023;472:145061.
Publication types
LinkOut - more resources
Full Text Sources

