Clinical characteristics and somatic burden of patients with mucopolysaccharidosis II with or without neurological involvement: An analysis from the Hunter Outcome Survey
- PMID: 38053935
- PMCID: PMC10694755
- DOI: 10.1016/j.ymgmr.2023.101005
Clinical characteristics and somatic burden of patients with mucopolysaccharidosis II with or without neurological involvement: An analysis from the Hunter Outcome Survey
Abstract
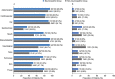
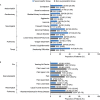
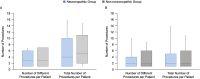
Approximately two-thirds of patients with mucopolysaccharidosis II (MPS II; Hunter syndrome) have neuronopathic disease, with central nervous system involvement; one-third have non-neuronopathic disease. This analysis of data from the Hunter Outcome Survey (HOS) compared the clinical manifestations and surgical and nonsurgical procedure history in patients with neuronopathic or non-neuronopathic MPS II. Prospective patients were identified in July 2018 in HOS for inclusion in this analysis as those with stable cognitive impairment status as assessed at 10 years of age and at a minimum of one follow-up visit at 11 to <20 years of age. Patients were stratified according to cognitive impairment status at 10 years into neuronopathic and non-neuronopathic groups, and clinical manifestations and surgical and nonsurgical procedure history were compared between the two groups. In total, 193 patients had cognitive impairment status assessments available (at 10 years and 11 to <20 years of age), 151 of whom had stable cognitive impairment status and were included; 100/151 (66.2%) were in the neuronopathic group and 51/151 (33.8%) in the non-neuronopathic group. The proportion of patients demonstrating manifestations by system organ class and the number of surgical and nonsurgical procedures per patient were broadly comparable in the neuronopathic and non-neuronopathic groups both before and after patients' 10th birthdays. The most common manifestations before patients' 10th birthdays, including facial features, joint stiffness and limited function, and hepatomegaly were reported in >80% of patients in both groups. For the neuronopathic and non-neuronopathic groups, the median [10th percentile, 90th percentile] number of different types of surgical and nonsurgical procedures per patient (3 [1, 6] and 3 [1, 7], respectively) and of all procedures per patient (4 [1, 10] and 5 [2, 11], respectively) before patients' 10th birthdays were similar, although the type of procedure may have differed. Thus, in the first two decades of life, patients with non-neuronopathic disease were found to have similar somatic manifestations to those of the neuronopathic group and undergo procedures for complications as often as those with neuronopathic disease.
Keywords: Enzyme replacement therapy; Hunter Outcome Survey; Mucopolysaccharidosis type II; Neuronopathic phenotype; Non-neuronopathic phenotype; Surgery.
© 2023 The Authors.
Conflict of interest statement
Heather Lau has received consulting fees from Amicus Therapeutics, Pfizer, Sanofi Genzyme, Takeda, and Taysha and research grants from Amicus Therapeutics, BioMarin, Pfizer, Protalix Biotherapeutics, Sangamo Therapeutics, Sanofi Genzyme, Takeda, and Ultragenyx, and is a full-time employee and stockholder of Ultragenyx Pharmaceutical. Paul Harmatz has received honoraria, consulting fees, and/or research grants from Adrenas Aeglea, Allievex, Amicus Therapeutics, Ascendis Pharma, Aspa therapeutics, Astellas Gene Therapies, AVROBIO, Azafaros, BioMarin, Calcilytix, Capsida Biotherapeutics, Chiesi, Denali Therapeutics, EdiGene, Grace Science, Homology Medicines, Inventiva Pharma, JCR Pharmaceuticals, Novel Pharma, Orchard Therapeutics, Orphazyme, Paradigm, QED Therapeutics, RallyBio, REGENXBIO, Renoviron, Sangamo Therapeutics, SalioGen Therapeutics, Sanofi Genzyme, Sobi and Takeda. Bianca Link has received fees and travel grants from Alexion, Sanofi Genzyme, Shire, and Takeda. Jaco Botha is a full-time employee of Takeda Pharmaceuticals International AG and stockholder of Takeda Pharmaceutical Company Limited. Jennifer Audi was a full-time employee of Takeda Pharmaceuticals International AG (now with Ultragenyx Europe GmbH) and was a stockholder of Takeda Pharmaceutical Company Limited.
Figures


References
-
- Neufeld E., Muenzer J. In: The Metabolic and Molecular Bases of Inherited Disease. Scriver C., Beaudet A., Sly W., Valle D., editors. McGraw-Hill; New York: 2001. The mucopolysaccharidoses; pp. 3421–3452.
-
- Wraith J.E., Scarpa M., Beck M., Bodamer O.A., De Meirleir L., Guffon N., Meldgaard Lund A., Malm G., Van der Ploeg A.T., Zeman J. Mucopolysaccharidosis type II (hunter syndrome): a clinical review and recommendations for treatment in the era of enzyme replacement therapy. Eur. J. Pediatr. 2008;167:267–277. - PMC - PubMed
-
- Kampmann C., Beck M., Morin I., Loehr J.P. Prevalence and characterization of cardiac involvement in hunter syndrome. J. Pediatr. 2011;159(327–331) - PubMed

