Automated MUltiscale simulation environment
- PMID: 38054103
- PMCID: PMC10694852
- DOI: 10.1039/d3dd00163f
Automated MUltiscale simulation environment
Abstract
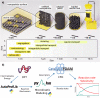
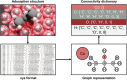
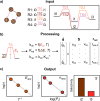
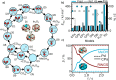
Multiscale techniques integrating detailed atomistic information on materials and reactions to predict the performance of heterogeneous catalytic full-scale reactors have been suggested but lack seamless implementation. The largest challenges in the multiscale modeling of reactors can be grouped into two main categories: catalytic complexity and the difference between time and length scales of chemical and transport phenomena. Here we introduce the Automated MUltiscale Simulation Environment AMUSE, a workflow that starts from Density Functional Theory (DFT) data, automates the analysis of the reaction networks through graph theory, prepares it for microkinetic modeling, and subsequently integrates the results into a standard open-source Computational Fluid Dynamics (CFD) code. We demonstrate the capabilities of AMUSE by applying it to the unimolecular iso-propanol dehydrogenation reaction and then, increasing the complexity, to the pre-commercial Pd/In2O3 catalyst employed for the CO2 hydrogenation to methanol. The results show that AMUSE allows the computational investigation of heterogeneous catalytic reactions in a comprehensive way, providing essential information for catalyst design from the atomistic to the reactor scale level.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

References
-
- Chorkendorff I. and Niemantsverdriet J. W., Concepts of Modern Catalysis and Kinetics, Wiley, Weinheim, 2003
-
- Salciccioli M. Stamatakis M. Caratzoulas S. Vlachos D. G. A review of multiscale modeling of metal-catalyzed reactions: Mechanism development for complexity and emergent behavior. Chem. Eng. Sci. 2011;66:4319–4355. doi: 10.1016/j.ces.2011.05.050. - DOI
-
- Bruix A. Margraf J. T. Andersen M. Reuter K. First-principles-based multiscale modelling of heterogeneous catalysis. Nat. Catal. 2019;2:659–670. doi: 10.1038/s41929-019-0298-3. - DOI
-
- Margraf J. T. Jung H. Scheurer C. Reuter K. Exploring catalytic reaction networks with machine learning. Nat. Catal. 2023;6:1–10. doi: 10.1038/s41929-023-00921-8. - DOI
-
- Wehinger G. D. Ambrosetti M. Cheula R. Ding Z.-B. Isoz M. Kreitz B. Kuhlmann K. Kutscherauer M. Niyogi K. Poissonnier J. et al., Quo vadis multiscale modeling in reaction engineering?–A perspective. Chem. Eng. Res. Des. 2022;184:39–58. doi: 10.1016/j.cherd.2022.05.030. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
