Respiratory distress syndrome prediction at birth by optical skin maturity assessment and machine learning models for limited-resource settings: a development and validation study
- PMID: 38054190
- PMCID: PMC10694507
- DOI: 10.3389/fped.2023.1264527
Respiratory distress syndrome prediction at birth by optical skin maturity assessment and machine learning models for limited-resource settings: a development and validation study
Abstract
Background: A handheld optical device was developed to evaluate a newborn's skin maturity by assessing the photobiological properties of the tissue and processing it with other variables to predict early neonatal prognosis related to prematurity. This study assessed the device's ability to predict respiratory distress syndrome (RDS).
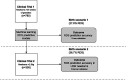
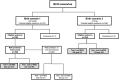
Methods: To assess the device's utility we enrolled newborns at childbirth in six urban perinatal centers from two multicenter single-blinded clinical trials. All newborns had inpatient follow-up until 72 h of life. We trained supervised machine learning models with data from 780 newborns in a Brazilian trial and provided external validation with data from 305 low-birth-weight newborns from another trial that assessed Brazilian and Mozambican newborns. The index test measured skin optical reflection with an optical sensor and adjusted acquired values with clinical variables such as birth weight and prenatal corticoid exposition for lung maturity, maternal diabetes, and hypertensive disturbances. The performance of the models was evaluated using intrasample k-parts cross-validation and external validation in an independent sample.
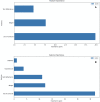
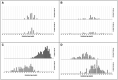
Results: Models adjusting three predictors (skin reflection, birth weight, and antenatal corticoid exposure) or five predictors had a similar performance, including or not maternal diabetes and hypertensive diseases. The best global accuracy was 89.7 (95% CI: 87.4 to 91.8, with a high sensitivity of 85.6% (80.2 to 90.0) and specificity of 91.3% (95% CI: 88.7 to 93.5). The test correctly discriminated RDS newborns in external validation, with 82.3% (95% CI: 77.5 to 86.4) accuracy. Our findings demonstrate a new way to assess a newborn's lung maturity, providing potential opportunities for earlier and more effective care.
Trial registration: RBR-3f5bm5 (online access: http://www.ensaiosclinicos.gov.br/rg/RBR-3f5bm5/), and RBR-33mjf (online access: https://ensaiosclinicos.gov.br/rg/RBR-33rnjf/).
Keywords: childbirth; equipment and supplies; machine learning; medical device; newborn; prematurity; respiratory distress syndrome; skin physiological phenomena.
© 2023 Reis, Pappa, Nader, do Vale, Silveira Neves, Vitral, Mussagy, Norberto Dias and Romanelli.
Conflict of interest statement
The authors declare a patent deposit on behalf of the Universidade Federal de Minas Gerais and Fundação de Amparo a Pesquisa de Minas Gerais, Brazil (http://www.fapemig.br/en/). BR1020170235688 (CTIT-PN862).
Figures

Similar articles
-
Assessment of Skin Maturity by LED Light at Birth and Its Association With Lung Maturity: Clinical Trial Secondary Outcomes.JMIR Biomed Eng. 2023 Dec 25;8:e52468. doi: 10.2196/52468. JMIR Biomed Eng. 2023. PMID: 38875690 Free PMC article.
-
Premature or Small for Gestational Age Discrimination: International Multicenter Trial Protocol for Classification of the Low-Birth-Weight Newborn Through the Optical Properties of the Skin.JMIR Res Protoc. 2020 Jul 14;9(7):e16477. doi: 10.2196/16477. JMIR Res Protoc. 2020. PMID: 32673275 Free PMC article.
-
Newborn Skin Maturity Medical Device Validation for Gestational Age Prediction: Clinical Trial.J Med Internet Res. 2022 Sep 7;24(9):e38727. doi: 10.2196/38727. J Med Internet Res. 2022. PMID: 36069805 Free PMC article.
-
Strategies for optimising antenatal corticosteroid administration for women with anticipated preterm birth.Cochrane Database Syst Rev. 2020 May 26;5(5):CD013633. doi: 10.1002/14651858.CD013633. Cochrane Database Syst Rev. 2020. PMID: 32452555 Free PMC article.
-
Corticosteroids for preventing neonatal respiratory morbidity after elective caesarean section at term.Cochrane Database Syst Rev. 2018 Aug 3;8(8):CD006614. doi: 10.1002/14651858.CD006614.pub3. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2021 Dec 22;12:CD006614. doi: 10.1002/14651858.CD006614.pub4. PMID: 30075059 Free PMC article. Updated.
References
-
- World Health Organization. Preterm birth. (2023). https://www.who.int/news-room/fact-sheets/detail/preterm-birth (Accessed May 31, 2023).
-
- Kumaran G, Sethu G, Ganapathy D. Respiratory distress syndrome in infants-an overview. PalArch's J Archaeol Egypt/Egyptol. (2020) 17(7):1902–11. Available from: https://archives.palarch.nl/index.php/jae/article/view/1432
LinkOut - more resources
Full Text Sources

