Belgian Recommendations for Analytical Verification and Validation of Immunohistochemical Tests in Laboratories of Anatomic Pathology
- PMID: 38054253
- PMCID: PMC10695338
- DOI: 10.1097/PAI.0000000000001165
Belgian Recommendations for Analytical Verification and Validation of Immunohistochemical Tests in Laboratories of Anatomic Pathology
Abstract
Analytical verification and validation of immunohistochemical (IHC) tests and their equipment are common practices for today's anatomic pathology laboratories. Few references or guidelines are available on how this should be performed. The study of Sciensano (the Belgian national competent authority regarding licensing of medical laboratories) performed in 2016, demonstrated a significant interlaboratory variation in validation procedures of IHC tests among Belgian laboratories. These results suggest the unavailability of practical information on the approach to the verification and validation of these tests. The existing Belgian Practice Guideline for the implementation of a quality management system in anatomic pathology laboratories has been reviewed to meet this demand and, in addition, to prepare the laboratories for the EU-IVD revised regulations (IVDR). This paper describes Belgian recommendations for the verification and validation of IHC tests before implementation, for ongoing validation, and for revalidation. For each type of test (according to the IVDR classification and the origin) and its intended use (purpose), it addresses how to perform analytical verification/validation by recommending: (1) the number of cases in the validation set, (2) the performance characteristics to be evaluated, (3) the objective acceptance criteria, (4) the evaluation method for the obtained results, and (5) how and when to revalidate. A literature study and a risk analysis taking into account the majority of variables regarding verification/validation of methods have been performed, resulting in an expert consensus recommendation that is a compromise among achievability, affordability, and patient safety. This new consensus recommendation has been incorporated in the aforementioned ISO 15189:2012-based Practice Guideline.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
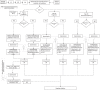
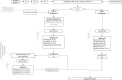

References
-
- Belgian Commission of Anatomic Pathology. https://www.sciensano.be/fr/biblio/directive-pratique-anatomie-pathologique Practice Guideline for setting up a quality system in laboratories for anatomic pathology in the context of the licensing decree. Accessed November 7, 2022:
-
- Verbeke H, Dierick AM, Ghislain V, et al. . Analytical validation of tests in laboratories of anatomic pathology: a Belgian population-based study. Accred Qual Assur. 2020;25:69–79.
-
- Fitzgibbons PL, Bradley LA, Fatheree LA, et al. . Principles of analytic validation of immunohistochemical assays: guideline from the College of American Pathologists Pathology and Laboratory Quality Center. Arch Pathol Lab Med. 2014;138:1432–1443. - PubMed
-
- Rabenau HF, Kessler HH, Kortenbusch M, et al. . Verification and validation of diagnostic laboratory tests in clinical virology. J Clin Virol. 2007;40:93–98. - PubMed

