Pathogenicity and virulence of Acinetobacter baumannii: Factors contributing to the fitness in healthcare settings and the infected host
- PMID: 38054753
- PMCID: PMC10732645
- DOI: 10.1080/21505594.2023.2289769
Pathogenicity and virulence of Acinetobacter baumannii: Factors contributing to the fitness in healthcare settings and the infected host
Abstract
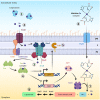
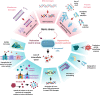
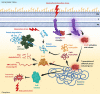
Acinetobacter baumannii is a common cause of healthcare-associated infections and hospital outbreaks, particularly in intensive care units. Much of the success of A. baumannii relies on its genomic plasticity, which allows rapid adaptation to adversity and stress. The capacity to acquire novel antibiotic resistance determinants and the tolerance to stresses encountered in the hospital environment promote A. baumannii spread among patients and long-term contamination of the healthcare setting. This review explores virulence factors and physiological traits contributing to A. baumannii infection and adaptation to the hospital environment. Several cell-associated and secreted virulence factors involved in A. baumannii biofilm formation, cell adhesion, invasion, and persistence in the host, as well as resistance to xeric stress imposed by the healthcare settings, are illustrated to give reasons for the success of A. baumannii as a hospital pathogen.
Keywords: Acinetobacter baumannii; desiccation; metals uptake; secretion systems; tolerance; virulence factors.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



References
-
- Whiteway C, Breine A, Philippe C, et al. Acinetobacter baumannii. Trends Microbiol. 2022;30(2):199–200. - PubMed
-
- AIDA Study Group, Dickstein Y, Lellouche J, Ben Dalak Amar M, et al. Treatment outcomes of colistin- and carbapenem-resistant Acinetobacter baumannii infections: an exploratory subgroup analysis of a randomized clinical trial. Clin Infect Dis. 2019;69(5):769–776. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
