Risk of Paradoxical Eczema in Patients Receiving Biologics for Psoriasis
- PMID: 38055239
- PMCID: PMC10701661
- DOI: 10.1001/jamadermatol.2023.4846
Risk of Paradoxical Eczema in Patients Receiving Biologics for Psoriasis
Abstract
Importance: Biologics used for plaque psoriasis have been reported to be associated with an atopic dermatitis (AD) phenotype, or paradoxical eczema, in some patients. The risk factors for this are unknown.
Objective: To explore risk of paradoxical eczema by biologic class and identify factors associated with paradoxical eczema.
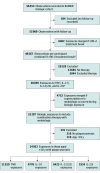
Design, setting, and participants: This prospective cohort study used data from the British Association of Dermatologists Biologics and Immunomodulators Register for adults treated with biologics for plaque psoriasis who were seen at multicenter dermatology clinics in the UK and Ireland. Included participants were registered and had 1 or more follow-up visits between September 2007 and December 2022.
Exposures: Duration of exposure to tumor necrosis factor (TNF) inhibitors, interleukin (IL) 17 inhibitors, IL-12/23 inhibitors, or IL-23 inhibitors until paradoxical eczema onset, treatment discontinuation, last follow-up, or death.
Main outcomes and measures: Incidence rates of paradoxical eczema, paradoxical eczema risk by biologic class, and the association of demographic and clinical variables with risk of paradoxical eczema were assessed using propensity score-weighted Cox proportional hazards regression models.
Results: Of 56 553 drug exposures considered, 24 997 from 13 699 participants were included. The 24 997 included exposures (median age, 46 years [IQR, 36-55 years]; 57% male) accrued a total exposure time of 81 441 patient-years. A total of 273 exposures (1%) were associated with paradoxical eczema. The adjusted incidence rates were 1.22 per 100 000 person-years for IL-17 inhibitors, 0.94 per 100 000 person-years for TNF inhibitors, 0.80 per 100 000 person-years for IL-12/23 inhibitors, and 0.56 per 100 000 person-years for IL-23 inhibitors. Compared with TNF inhibitors, IL-23 inhibitors were associated with a lower risk of paradoxical eczema (hazard ratio [HR], 0.39; 95% CI, 0.19-0.81), and there was no association of IL-17 inhibitors (HR, 1.03; 95% CI, 0.74-1.42) or IL-12/23 inhibitors (HR, 0.87; 95% CI, 0.66-1.16) with risk of paradoxical eczema. Increasing age (HR, 1.02 per year; 95% CI, 1.01-1.03) and history of AD (HR, 12.40; 95% CI, 6.97-22.06) or hay fever (HR, 3.78; 95% CI, 1.49-9.53) were associated with higher risk of paradoxical eczema. There was a lower risk in males (HR, 0.60; 95% CI, 0.45-0.78).
Conclusions and relevance: In this study, in biologic-treated patients with psoriasis, paradoxical eczema risk was lowest in patients receiving IL-23 inhibitors. Increasing age, female sex, and history of AD or hay fever were associated with higher risk of paradoxical eczema. The overall incidence of paradoxical eczema was low. Further study is needed to replicate these findings.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

