Clinical Outcomes of Magnetic Seizure Therapy vs Electroconvulsive Therapy for Major Depressive Episode: A Randomized Clinical Trial
- PMID: 38055283
- PMCID: PMC10701670
- DOI: 10.1001/jamapsychiatry.2023.4599
Clinical Outcomes of Magnetic Seizure Therapy vs Electroconvulsive Therapy for Major Depressive Episode: A Randomized Clinical Trial
Abstract
Importance: Electroconvulsive therapy (ECT) is highly effective and rapid in treating depression, but it carries a risk of significant cognitive adverse effects. Magnetic seizure therapy (MST), an investigational antidepressant treatment, may maintain the robust antidepressant efficacy of ECT while substantially reducing adverse effects due to its enhanced focality and weaker stimulation strength; however, previous clinical trials of MST were limited by small sample sizes.
Objective: To compare the antidepressant efficacy of MST vs ultrabrief pulse right unilateral (RUL) ECT.
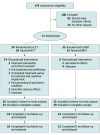
Design, setting, and participants: A between-participants, double-blinded, randomized clinical trial was conducted at 3 academic hospitals from June 2007 to August 2012. Adults aged 18 to 90 years who were referred for treatment with ECT, had a major depressive episode in the context of major depressive disorder or bipolar disorder, and had a baseline 24-item Hamilton Depression Rating Scale (HDRS-24) total score of 18 or higher were included. Participants were randomly assigned 1:1 to treatment with MST or ultrabrief pulse RUL ECT. After the treatment course, patients were naturalistically followed up for up to 6 months to examine the durability of clinical effects.
Interventions: Treatment with MST, applied at 100 Hz at 100% of the maximum device power for 10 seconds, or ultrabrief pulse RUL ECT, applied at 6 times seizure threshold.
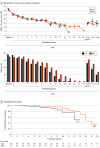
Main outcomes and measures: The primary outcome was change from baseline in HDRS-24 total score, with patients followed up for up to 6 months. A reduction of at least 50% in the HDRS-24 score indicated response, and at least a 60% decrease in the HDRS-24 score and a total score of 8 or less indicated remission.
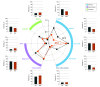
Results: Of the 73 participants (41 [56.2%] female; mean [SD] age, 48 [14.1] years), 35 were randomized to MST and 38 to ECT. Among them, 53 (72.6%) were classified as completers (29 in the MST group and 24 in the ECT group). Both MST and ECT demonstrated clinically meaningful antidepressant effects. In the intent-to-treat sample, 18 participants (51.4%) in the MST group and 16 (42.1%) in the ECT group met response criteria; 13 (37.1%) in the MST group and 10 (26.3%) in the ECT group met remission criteria. Among completers, 17 of 29 (58.6%) in the MST group and 15 of 24 (62.5%) in the ECT group met response criteria; 13 of 29 (44.8%) in the MST group and 10 of 24 (41.7%) in the ECT group met remission criteria. There was no significant difference between MST and ECT for either response or remission rates. However, the mean (SD) number of treatments needed to achieve remission was 9.0 (3.1) with MST and 6.7 (3.3) with ECT, a difference of 2.3 treatments (t71.0 = 3.1; P = .003). Both MST and ECT showed a sustained benefit over a 6-month follow-up period, again with no significant difference between them. Compared with MST, ECT had significantly longer time to orientation after treatment (threshold level: F1,56 = 10.0; P = .003) and greater severity of subjective adverse effects, particularly in the physical and cognitive domains.
Conclusions and relevance: This randomized clinical trial found that the efficacy of MST was indistinguishable from that of ultrabrief pulse RUL ECT, the safest form of ECT currently available. These results support the continued development of MST and provide evidence for advantages relative to state-of-the-art ECT.
Trial registration: ClinicalTrials.gov Identifier: NCT00488748.
Conflict of interest statement
Figures

References
-
- World Health Organization . Depression and other common mental disorders: global health estimates. Accessed March 15, 2023. https://iris.who.int/handle/10665/254610
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

