Discovery and characterization of cross-reactive intrahepatic antibodies in severe alcoholic hepatitis
- PMID: 38055614
- PMCID: PMC10699809
- DOI: 10.7554/eLife.86678
Discovery and characterization of cross-reactive intrahepatic antibodies in severe alcoholic hepatitis
Abstract
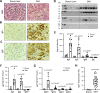
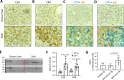
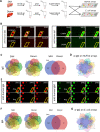
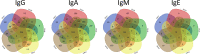
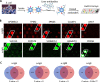
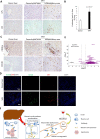
The pathogenesis of antibodies in severe alcoholic hepatitis (SAH) remains unknown. We analyzed immunoglobulins (Ig) in explanted livers from SAH patients (n=45) undergoing liver transplantation and tissues from corresponding healthy donors (HD, n=10) and found massive deposition of IgG and IgA isotype antibodies associated with complement fragment C3d and C4d staining in ballooned hepatocytes in SAH livers. Ig extracted from SAH livers, but not patient serum exhibited hepatocyte killing efficacy. Employing human and Escherichia coli K12 proteome arrays, we profiled the antibodies extracted from explanted SAH, livers with other diseases, and HD livers. Compared with their counterparts extracted from livers with other diseases and HD, antibodies of IgG and IgA isotypes were highly accumulated in SAH and recognized a unique set of human proteins and E. coli antigens. Further, both Ig- and E. coli-captured Ig from SAH livers recognized common autoantigens enriched in several cellular components including cytosol and cytoplasm (IgG and IgA), nucleus, mitochondrion, and focal adhesion (IgG). Except IgM from primary biliary cholangitis livers, no common autoantigen was recognized by Ig- and E. coli-captured Ig from livers with other diseases. These findings demonstrate the presence of cross-reacting anti-bacterial IgG and IgA autoantibodies in SAH livers.
Keywords: E. coli; E. coli arrays; IgA; IgG; alcoholic hepatitis; autoantibodies; human; immunology; inflammation.
Conflict of interest statement
AA, GS, TG, JM, XH, MH, AC, RW, BP, SO, EK, AG, LQ, BP, JB, RA, ZZ, HL, DF, CC, JQ, BG, HZ, ZS No competing interests declared
Figures







Update of
-
Discovery and characterization of cross-reactive intrahepatic antibodies in severe alcoholic hepatitis.bioRxiv [Preprint]. 2023 Feb 23:2023.02.23.529702. doi: 10.1101/2023.02.23.529702. bioRxiv. 2023. Update in: Elife. 2023 Dec 06;12:RP86678. doi: 10.7554/eLife.86678. PMID: 36865259 Free PMC article. Updated. Preprint.
References
-
- Bogdanos DP, Baum H, Grasso A, Okamoto M, Butler P, Ma Y, Rigopoulou E, Montalto P, Davies ET, Burroughs AK, Vergani D. Microbial mimics are major targets of crossreactivity with human pyruvate dehydrogenase in primary biliary cirrhosis. Journal of Hepatology. 2004;40:31–39. doi: 10.1016/s0168-8278(03)00501-4. - DOI - PubMed
-
- Chen CS, Sullivan S, Anderson T, Tan AC, Alex PJ, Brant SR, Cuffari C, Bayless TM, Talor MV, Burek CL, Wang H, Li R, Datta LW, Wu Y, Winslow RL, Zhu H, Li X. Identification of novel serological biomarkers for inflammatory bowel disease using Escherichia coli proteome chip. Molecular & Cellular Proteomics. 2009;8:1765–1776. doi: 10.1074/mcp.M800593-MCP200. - DOI - PMC - PubMed
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

