IL-2 produced by HBV-specific T cells as a biomarker of viral control and predictor of response to PD-1 therapy across clinical phases of chronic hepatitis B
- PMID: 38055623
- PMCID: PMC10984660
- DOI: 10.1097/HC9.0000000000000337
IL-2 produced by HBV-specific T cells as a biomarker of viral control and predictor of response to PD-1 therapy across clinical phases of chronic hepatitis B
Abstract
Background: There are no immunological biomarkers that predict control of chronic hepatitis B (CHB). The lack of immune biomarkers raises concerns for therapies targeting PD-1/PD-L1 because they have the potential for immune-related adverse events. Defining specific immune functions associated with control of HBV replication could identify patients likely to respond to anti-PD-1/PD-L1 therapies and achieve a durable functional cure.
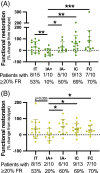
Methods: We enrolled immunotolerant, HBeAg+ immune-active (IA+), HBeAg- immune-active (IA-), inactive carriers, and functionally cured patients to test ex vivo PD-1 blockade on HBV-specific T cell functionality. Peripheral blood mononuclear cells were stimulated with overlapping peptides covering HBV proteins +/-α-PD-1 blockade. Functional T cells were measured using a 2-color FluoroSpot assay for interferon-γ and IL-2. Ex vivo functional restoration was compared to the interferon response capacity assay, which predicts overall survival in cancer patients receiving checkpoint inhibitors.
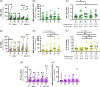
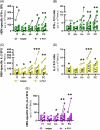
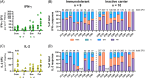
Results: Ex vivo interferon-γ+ responses did not differ across clinical phases. IL-2+ responses were significantly higher in patients with better viral control and preferentially restored with PD-1 blockade. Inactive carrier patients displayed the greatest increase in IL-2 production, which was dominated by CD4 T cell and response to the HBcAg. The interferon response capacity assay significantly correlated with the degree of HBV-specific T cell restoration.
Conclusions: IL-2 production was associated with better HBV control and superior to interferon-γ as a marker of T cell restoration following ex vivo PD-1 blockade. Our study suggests that responsiveness to ex vivo PD-1 blockade, or the interferon response capacity assay, may support stratification for α-PD-1 therapies.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Association for the Study of Liver Diseases.
Conflict of interest statement
The authors declare that this study was funded in part by AstraZeneca. Authors Sara Ferrando-Martinez and Scott H Robins were employed by AstraZeneca. Jordan J Feld receives research funding by Abbvie, Arbutus Biopharma, Gilead Sciences Inc., Janssen Pharmaceuticals, Eiger Biopharmaceuticals, and Enanta Pharmaceuticals; and reports compensation from consulting/scientific advising for Abbvie, Arbutus Biopharma, Gilead Sciences Inc., and GlaxoSmithKline. Scott Fung receives research funding by Gilead Sciences Inc.; and reports compensation from consulting/scientific advising for Gilead Sciences Inc., Abbvie, Janssen Pharmaceuticals, Assembly Biosciences. Harry L.A. Janssen receives research funding by Abbvie, Gilead Sciences Inc., GlaxoSmithKline, Janssen Pharmaceuticals, Roche, Vir Biotechnology; and reports compensation from consulting/scientific advising for ALIGOS Therapeutics, Antios Therapeutics, Arbutus Biopharma, Eiger Biopharmaceuticals, Gilead Sciences Inc., GlaxoSmithKline, Janssen Pharmaceuticals, Merck, Roche, VBI Vaccines Inc., Vir Biotechnology, and Viroclinics Biosciences. Adam J. Gehring. receives research funding from Janssen Pharmaceuticals, GlaxoSmithKline, and Gilead Sciences Inc. and reports compensation from consulting/scientific advising: Janssen Pharmaceuticals, Roche, GlaxoSmithKline, Vir Biotechnology. David G. Brooks holds the US Patent (PCT/CA2022/051519) for the Interferon response capacity assay. The remaining authors have no conflicts to report.
Figures



References
-
- Alatrakchi N, Koziel MJ. Antiviral T-cell responses and therapy in chronic hepatitis B. J Hepatol. 2003;39:631–4. - PubMed
-
- Boni C, Laccabue D, Lampertico P, Giuberti T, Viganò M, Schivazappa S, et al. . Restored function of HBV-specific T cells after long-term effective therapy with nucleos(t)ide analogues. Gastroenterology. 2012;143:963–73.e9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

