Current Practices in LC-MS Untargeted Metabolomics: A Scoping Review on the Use of Pooled Quality Control Samples
- PMID: 38055671
- PMCID: PMC10753522
- DOI: 10.1021/acs.analchem.3c02924
Current Practices in LC-MS Untargeted Metabolomics: A Scoping Review on the Use of Pooled Quality Control Samples
Abstract
Untargeted metabolomics is an analytical approach with numerous applications serving as an effective metabolic phenotyping platform to characterize small molecules within a biological system. Data quality can be challenging to evaluate and demonstrate in metabolomics experiments. This has driven the use of pooled quality control (QC) samples for monitoring and, if necessary, correcting for analytical variance introduced during sample preparation and data acquisition stages. Described herein is a scoping literature review detailing the use of pooled QC samples in published untargeted liquid chromatography-mass spectrometry (LC-MS) based metabolomics studies. A literature query was performed, the list of papers was filtered, and suitable articles were randomly sampled. In total, 109 papers were each reviewed by at least five reviewers, answering predefined questions surrounding the use of pooled quality control samples. The results of the review indicate that use of pooled QC samples has been relatively widely adopted by the metabolomics community and that it is used at a similar frequency across biological taxa and sample types in both small- and large-scale studies. However, while many studies generated and analyzed pooled QC samples, relatively few reported the use of pooled QC samples to improve data quality. This demonstrates a clear opportunity for the field to more frequently utilize pooled QC samples for quality reporting, feature filtering, analytical drift correction, and metabolite annotation. Additionally, our survey approach enabled us to assess the ambiguity in the reporting of the methods used to describe the generation and use of pooled QC samples. This analysis indicates that many details of the QC framework are missing or unclear, limiting the reader's ability to determine which QC steps have been taken. Collectively, these results capture the current state of pooled QC sample usage and highlight existing strengths and deficiencies as they are applied in untargeted LC-MS metabolomics.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


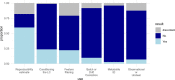
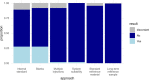
References
-
- Kirwan J. A.; Gika H.; Beger R. D.; Bearden D.; Dunn W. B.; Goodacre R.; Theodoridis G.; Witting M.; Yu L.-R.; Wilson I. D. the metabolomics Quality Assurance and Quality Control Consortium (mQACC). Quality Assurance and Quality Control Reporting in Untargeted Metabolic Phenotyping: mQACC Recommendations for Analytical Quality Management. Metabolomics 2022, 18 (9), 70. 10.1007/s11306-022-01926-3. - DOI - PMC - PubMed
-
- Beger R. D.; Dunn W. B.; Bandukwala A.; Bethan B.; Broadhurst D.; Clish C. B.; Dasari S.; Derr L.; Evans A.; Fischer S.; Flynn T.; Hartung T.; Herrington D.; Higashi R.; Hsu P.-C.; Jones C.; Kachman M.; Karuso H.; Kruppa G.; Lippa K.; Maruvada P.; Mosley J.; Ntai I.; O’Donovan C.; Playdon M.; Raftery D.; Shaughnessy D.; Souza A.; Spaeder T.; Spalholz B.; Tayyari F.; Ubhi B.; Verma M.; Walk T.; Wilson I.; Witkin K.; Bearden D. W.; Zanetti K. A. Towards Quality Assurance and Quality Control in Untargeted Metabolomics Studies. Metabolomics 2019, 15 (1), 4. 10.1007/s11306-018-1460-7. - DOI - PMC - PubMed
-
- Viant M. R.; Ebbels T. M. D.; Beger R. D.; Ekman D. R.; Epps D. J. T.; Kamp H.; Leonards P. E. G.; Loizou G. D.; MacRae J. I.; van Ravenzwaay B.; Rocca-Serra P.; Salek R. M.; Walk T.; Weber R. J. M. Use Cases, Best Practice and Reporting Standards for Metabolomics in Regulatory Toxicology. Nat. Commun. 2019, 10 (1), 3041. 10.1038/s41467-019-10900-y. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

