Transient CD4+ T cell depletion during suppressive ART reduces the HIV reservoir in humanized mice
- PMID: 38055722
- PMCID: PMC10699604
- DOI: 10.1371/journal.ppat.1011824
Transient CD4+ T cell depletion during suppressive ART reduces the HIV reservoir in humanized mice
Abstract
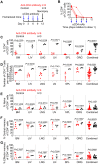
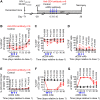
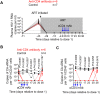
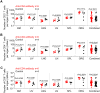
Lifelong treatment is required for people living with HIV as current antiretroviral therapy (ART) does not eradicate HIV infection. Latently infected cells are essentially indistinguishable from uninfected cells and cannot be depleted by currently available approaches. This study evaluated antibody mediated transient CD4+ T cell depletion as a strategy to reduce the latent HIV reservoir. Anti-CD4 antibodies effectively depleted CD4+ T cells in the peripheral blood and tissues of humanized mice. We then demonstrate that antibody-mediated CD4+ T cell depletion of HIV infected ART-suppressed animals results in substantial reductions in cell-associated viral RNA and DNA levels in peripheral blood cells over the course of anti-CD4 antibody treatment. Recovery of CD4+ T cells was observed in all tissues analyzed except for the lung 26 days after cessation of antibody treatment. After CD4+ T cell recovery, significantly lower levels of cell-associated viral RNA and DNA were detected in the tissues of anti-CD4 antibody-treated animals. Further, an 8.5-fold reduction in the levels of intact HIV proviral DNA and a 3.1-fold reduction in the number of latently infected cells were observed in anti-CD4-antibody-treated animals compared with controls. However, there was no delay in viral rebound when ART was discontinued in anti-CD4 antibody-treated animals following CD4+ T cell recovery compared with controls. Our results suggest that transient CD4+ T cell depletion, a long-standing clinical intervention that might have an acceptable safety profile, during suppressive ART can reduce the size of the HIV reservoir in humanized mice.
Copyright: © 2023 Ling et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Antibody-Mediated CD4 Depletion Induces Homeostatic CD4+ T Cell Proliferation without Detectable Virus Reactivation in Antiretroviral Therapy-Treated Simian Immunodeficiency Virus-Infected Macaques.J Virol. 2018 Oct 29;92(22):e01235-18. doi: 10.1128/JVI.01235-18. Print 2018 Nov 15. J Virol. 2018. PMID: 30185596 Free PMC article.
-
Establishment of a Novel Humanized Mouse Model To Investigate In Vivo Activation and Depletion of Patient-Derived HIV Latent Reservoirs.J Virol. 2019 Mar 5;93(6):e02051-18. doi: 10.1128/JVI.02051-18. Print 2019 Mar 15. J Virol. 2019. PMID: 30626677 Free PMC article.
-
Humanized Mouse Model of HIV-1 Latency with Enrichment of Latent Virus in PD-1+ and TIGIT+ CD4 T Cells.J Virol. 2019 May 1;93(10):e02086-18. doi: 10.1128/JVI.02086-18. Print 2019 May 15. J Virol. 2019. PMID: 30842333 Free PMC article.
-
Brain macrophages harbor latent, infectious simian immunodeficiency virus.AIDS. 2019 Dec 1;33 Suppl 2(Suppl 2):S181-S188. doi: 10.1097/QAD.0000000000002269. AIDS. 2019. PMID: 31789817 Free PMC article. Review.
-
HIV-1 reservoir dynamics in CD4+ T cells.Curr Opin HIV AIDS. 2019 Mar;14(2):108-114. doi: 10.1097/COH.0000000000000521. Curr Opin HIV AIDS. 2019. PMID: 30531293 Review.
Cited by
-
Association between gut microbiota in HIV-infected patients and immune reconstitution following antiretroviral therapy (ART).BMC Infect Dis. 2025 May 6;25(1):666. doi: 10.1186/s12879-025-10995-3. BMC Infect Dis. 2025. PMID: 40329177 Free PMC article.
-
Long-Term Human Immune Reconstitution, T-Cell Development, and Immune Reactivity in Mice Lacking the Murine Major Histocompatibility Complex: Validation with Cellular and Gene Expression Profiles.Cells. 2024 Oct 12;13(20):1686. doi: 10.3390/cells13201686. Cells. 2024. PMID: 39451205 Free PMC article.
-
Analysis of the effect of HDAC inhibitors on the formation of the HIV reservoir.mBio. 2024 Sep 11;15(9):e0163224. doi: 10.1128/mbio.01632-24. Epub 2024 Aug 13. mBio. 2024. PMID: 39136440 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

