CD5L is a canonical component of circulatory IgM
- PMID: 38055740
- PMCID: PMC10723121
- DOI: 10.1073/pnas.2311265120
CD5L is a canonical component of circulatory IgM
Abstract
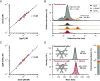
Immunoglobulin M (IgM) is an evolutionary conserved key component of humoral immunity, and the first antibody isotype to emerge during an immune response. IgM is a large (1 MDa), multimeric protein, for which both hexameric and pentameric structures have been described, the latter additionally containing a joining (J) chain. Using a combination of single-particle mass spectrometry and mass photometry, proteomics, and immunochemical assays, we here demonstrate that circulatory (serum) IgM exclusively exists as a complex of J-chain-containing pentamers covalently bound to the small (36 kDa) protein CD5 antigen-like (CD5L, also called apoptosis inhibitor of macrophage). In sharp contrast, secretory IgM in saliva and milk is principally devoid of CD5L. Unlike IgM itself, CD5L is not produced by B cells, implying that it associates with IgM in the extracellular space. We demonstrate that CD5L integration has functional implications, i.e., it diminishes IgM binding to two of its receptors, the FcαµR and the polymeric Immunoglobulin receptor. On the other hand, binding to FcµR as well as complement activation via C1q seem unaffected by CD5L integration. Taken together, we redefine the composition of circulatory IgM as a J-chain containing pentamer, always in complex with CD5L.
Keywords: CD5L; IgM; immunoglobulin; mass spectrometry; structure.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

