Seabirds boost coral reef resilience
- PMID: 38055814
- PMCID: PMC10699780
- DOI: 10.1126/sciadv.adj0390
Seabirds boost coral reef resilience
Abstract
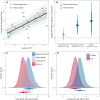
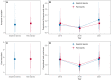
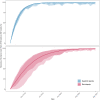
Global climate change threatens tropical coral reefs, yet local management can influence resilience. While increasing anthropogenic nutrients reduce coral resistance and recovery, it is unknown how the loss, or restoration, of natural nutrient flows affects reef recovery. Here, we test how natural seabird-derived nutrient subsidies, which are threatened by invasive rats, influence the mechanisms and patterns of reef recovery following an extreme marine heatwave using multiyear field experiments, repeated surveys, and Bayesian modeling. Corals transplanted from rat to seabird islands quickly assimilated seabird-derived nutrients, fully acclimating to new nutrient conditions within 3 years. Increased seabird-derived nutrients, in turn, caused a doubling of coral growth rates both within individuals and across entire reefs. Seabirds were also associated with faster recovery time of Acropora coral cover (<4 years) and more dynamic recovery trajectories of entire benthic communities. We conclude that restoring seabird populations and associated nutrient pathways may foster greater coral reef resilience through enhanced growth and recovery rates of corals.
Figures


References
-
- Intergovernmental Panel on Climate Change (IPCC), "Climate Change 2022: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change," H.-O. Pörtner, D.C. Roberts, M. Tignor, E.S. Poloczanska, K. Mintenbeck, A. Alegría, M. Craig, S. Langsdorf, S. Löschke, V. Möller, A. Okem, B. Rama, Eds. (Cambridge University Press) pp. 3056, doi: 10.1017/9781009325844. - DOI
-
- J. W. Moore, D. E. Schindler, Getting ahead of climate change for ecological adaptation and resilience. Science 376, 1421–1426 (2022). - PubMed
-
- T. P. Hughes, K. D. Anderson, S. R. Connolly, S. F. Heron, J. T. Kerry, J. M. Lough, A. H. Baird, J. K. Baum, M. L. Berumen, T. C. Bridge, D. C. Claar, C. M. Eakin, J. P. Gilmour, N. A. J. Graham, H. Harrison, J.-P. A. Hobbs, A. S. Hoey, M. Hoogenboom, R. J. Lowe, M. T. McCulloch, J. M. Pandolfi, M. Pratchett, V. Schoepf, G. Torda, S. K. Wilson, Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 359, 80–83 (2018). - PubMed
-
- C. G. Jones, J. H. Lawton, M. Shachak, Organisms as ecosystem engineers. Oikos 69, 373–386 (1994).
-
- A. C. Baker, P. W. Glynn, B. Riegl, Climate change and coral reef bleaching: An ecological assessment of long-term impacts, recovery trends and future outlook. Estuar. Coast. Shelf Sci. 80, 435–471 (2008).
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

