DECTIN-1: A modifier protein in CTLA-4 haploinsufficiency
- PMID: 38055819
- PMCID: PMC10699772
- DOI: 10.1126/sciadv.adi9566
DECTIN-1: A modifier protein in CTLA-4 haploinsufficiency
Abstract
Autosomal dominant loss-of-function (LoF) variants in cytotoxic T-lymphocyte associated protein 4 (CTLA4) cause immune dysregulation with autoimmunity, immunodeficiency and lymphoproliferation (IDAIL). Incomplete penetrance and variable expressivity are characteristic of IDAIL caused by CTLA-4 haploinsufficiency (CTLA-4h), pointing to a role for genetic modifiers. Here, we describe an IDAIL proband carrying a maternally inherited pathogenic CTLA4 variant and a paternally inherited rare LoF missense variant in CLEC7A, which encodes for the β-glucan pattern recognition receptor DECTIN-1. The CLEC7A variant led to a loss of DECTIN-1 dimerization and surface expression. Notably, DECTIN-1 stimulation promoted human and mouse regulatory T cell (Treg) differentiation from naïve αβ and γδ T cells, even in the absence of transforming growth factor-β. Consistent with DECTIN-1's Treg-boosting ability, partial DECTIN-1 deficiency exacerbated the Treg defect conferred by CTL4-4h. DECTIN-1/CLEC7A emerges as a modifier gene in CTLA-4h, increasing expressivity of CTLA4 variants and acting in functional epistasis with CTLA-4 to maintain immune homeostasis and tolerance.
Figures




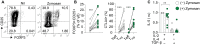

References
-
- D. Schubert, C. Bode, R. Kenefeck, T. Z. Hou, J. B. Wing, A. Kennedy, A. Bulashevska, B. S. Petersen, A. A. Schäffer, B. A. Grüning, S. Unger, N. Frede, U. Baumann, T. Witte, R. E. Schmidt, G. Dueckers, T. Niehues, S. Seneviratne, M. Kanariou, C. Speckmann, S. Ehl, A. Rensing-Ehl, K. Warnatz, M. Rakhmanov, R. Thimme, P. Hasselblatt, F. Emmerich, T. Cathomen, R. Backofen, P. Fisch, M. Seidl, A. May, A. Schmitt-Graeff, S. Ikemizu, U. Salzer, A. Franke, S. Sakaguchi, L. S. K. Walker, D. M. Sansom, B. Grimbacher, Autosomal dominant immune dysregulation syndrome in humans with CTLA4 mutations. Nat. Med. 20, 1410–1416 (2014). - PMC - PubMed
-
- H. S. Kuehn, W. Ouyang, B. Lo, E. K. Deenick, J. E. Niemela, D. T. Avery, J. N. Schickel, D. Q. Tran, J. Stoddard, Y. Zhang, D. M. Frucht, B. Dumitriu, P. Scheinberg, L. R. Folio, C. A. Frein, S. Price, C. Koh, T. Heller, C. M. Seroogy, A. Huttenlocher, V. K. Rao, H. C. Su, D. Kleiner, L. D. Notarangelo, Y. Rampertaap, K. N. Olivier, J. McElwee, J. Hughes, S. Pittaluga, J. B. Oliveira, E. Meffre, T. A. Fleisher, S. M. Holland, M. J. Lenardo, S. G. Tangye, G. Uzel, Immune dysregulation in human subjects with heterozygous germline mutations in CTLA4. Science 345, 1623–1627 (2014). - PMC - PubMed
-
- L. S. K. Walker, D. M. Sansom, The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat. Rev. Immunol. 11, 852–863 (2011). - PubMed
-
- V. Ovcinnikovs, E. M. Ross, L. Petersone, N. M. Edner, F. Heuts, E. Ntavli, A. Kogimtzis, A. Kennedy, C. J. Wang, C. L. Bennett, D. M. Sansom, L. S. K. Walker, CTLA-4-mediated transendocytosis of costimulatory molecules primarily targets migratory dendritic cells. Sci Immunol 4, (2019). - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

