A mechanochemical model recapitulates distinct vertebrate gastrulation modes
- PMID: 38055823
- PMCID: PMC10699781
- DOI: 10.1126/sciadv.adh8152
A mechanochemical model recapitulates distinct vertebrate gastrulation modes
Abstract
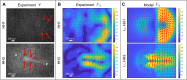
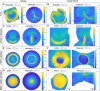
During vertebrate gastrulation, an embryo transforms from a layer of epithelial cells into a multilayered gastrula. This process requires the coordinated movements of hundreds to tens of thousands of cells, depending on the organism. In the chick embryo, patterns of actomyosin cables spanning several cells drive coordinated tissue flows. Here, we derive a minimal theoretical framework that couples actomyosin activity to global tissue flows. Our model predicts the onset and development of gastrulation flows in normal and experimentally perturbed chick embryos, mimicking different gastrulation modes as an active stress instability. Varying initial conditions and a parameter associated with active cell ingression, our model recapitulates distinct vertebrate gastrulation morphologies, consistent with recently published experiments in the chick embryo. Altogether, our results show how changes in the patterning of critical cell behaviors associated with different force-generating mechanisms contribute to distinct vertebrate gastrulation modes via a self-organizing mechanochemical process.
Figures


Similar articles
-
Cellular processes driving gastrulation in the avian embryo.Mech Dev. 2020 Sep;163:103624. doi: 10.1016/j.mod.2020.103624. Epub 2020 Jun 17. Mech Dev. 2020. PMID: 32562871 Free PMC article. Review.
-
Reconstruction of distinct vertebrate gastrulation modes via modulation of key cell behaviors in the chick embryo.Sci Adv. 2023 Jan 4;9(1):eabn5429. doi: 10.1126/sciadv.abn5429. Epub 2023 Jan 4. Sci Adv. 2023. PMID: 36598979 Free PMC article.
-
Control of tissue flows and embryo geometry in avian gastrulation.Nat Commun. 2025 Jun 4;16(1):5174. doi: 10.1038/s41467-025-60249-8. Nat Commun. 2025. PMID: 40467548 Free PMC article.
-
Decoupling of amniote gastrulation and streak formation reveals a morphogenetic unity in vertebrate mesoderm induction.Development. 2013 Jul;140(13):2691-6. doi: 10.1242/dev.094318. Epub 2013 May 22. Development. 2013. PMID: 23698348
-
Conserved patterns of cell movements during vertebrate gastrulation.Curr Biol. 2005 Mar 29;15(6):R213-28. doi: 10.1016/j.cub.2005.03.016. Curr Biol. 2005. PMID: 15797016 Review.
Cited by
-
Emergence of cellular nematic order is a conserved feature of gastrulation in animal embryos.Nat Commun. 2025 Jul 1;16(1):5946. doi: 10.1038/s41467-025-61045-0. Nat Commun. 2025. PMID: 40595575 Free PMC article.
-
Dorsal lip maturation and initial archenteron extension depend on Wnt11 family ligands.Dev Biol. 2023 Jan;493:67-79. doi: 10.1016/j.ydbio.2022.10.013. Epub 2022 Nov 2. Dev Biol. 2023. PMID: 36334838 Free PMC article.
-
A chemo-mechanical model of endoderm movements driving elongation of the amniote hindgut.bioRxiv [Preprint]. 2023 May 18:2023.05.18.541363. doi: 10.1101/2023.05.18.541363. bioRxiv. 2023. Update in: Development. 2023 Nov 15;150(22):dev202010. doi: 10.1242/dev.202010. PMID: 37292966 Free PMC article. Updated. Preprint.
-
Emergence of cellular nematic order is a conserved feature of gastrulation in animal embryos.bioRxiv [Preprint]. 2025 May 15:2024.07.11.603175. doi: 10.1101/2024.07.11.603175. bioRxiv. 2025. Update in: Nat Commun. 2025 Jul 1;16(1):5946. doi: 10.1038/s41467-025-61045-0. PMID: 39071444 Free PMC article. Updated. Preprint.
-
Cyclic muscle contractions reinforce the actomyosin motors and mediate the full elongation of C. elegans embryo.Elife. 2024 Jun 20;12:RP90505. doi: 10.7554/eLife.90505. Elife. 2024. PMID: 38900560 Free PMC article.
References
-
- C. D. Stern, Gastrulation: From Cells to Embryo (CSHL Press, 2004).
-
- M. Saadaoui, D. Rocancourt, J. Roussel, F. Corson, J. Gros, A tensile ring drives tissue flows to shape the gastrulating amniote embryo. Science 367, 453–458 (2020). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

