A neuronal coping mechanism linking stress-induced anxiety to motivation for reward
- PMID: 38055830
- PMCID: PMC10699782
- DOI: 10.1126/sciadv.adh9620
A neuronal coping mechanism linking stress-induced anxiety to motivation for reward
Abstract
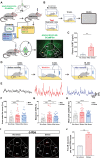
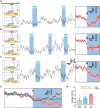
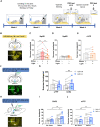
Stress coping involves innate and active motivational behaviors that reduce anxiety under stressful situations. However, the neuronal bases directly linking stress, anxiety, and motivation are largely unknown. Here, we show that acute stressors activate mouse GABAergic neurons in the interpeduncular nucleus (IPN). Stress-coping behavior including self-grooming and reward behavior including sucrose consumption inherently reduced IPN GABAergic neuron activity. Optogenetic silencing of IPN GABAergic neuron activation during acute stress episodes mimicked coping strategies and alleviated anxiety-like behavior. In a mouse model of stress-enhanced motivation for sucrose seeking, photoinhibition of IPN GABAergic neurons reduced stress-induced motivation for sucrose, whereas photoactivation of IPN GABAergic neurons or excitatory inputs from medial habenula potentiated sucrose seeking. Single-cell sequencing, fiber photometry, and optogenetic experiments revealed that stress-activated IPN GABAergic neurons that drive motivated sucrose seeking express somatostatin. Together, these data suggest that stress induces innate behaviors and motivates reward seeking to oppose IPN neuronal activation as an anxiolytic stress-coping mechanism.
Figures




References
-
- Chrousos G. P., Gold P. W., The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA 267, 1244–1252 (1992). - PubMed
-
- Tafet G. E., Nemeroff C. B., The links between stress and depression: Psychoneuroendocrinological, genetic, and environmental interactions. J. Neuropsychiatry Clin. Neurosci. 28, 77–88 (2016). - PubMed
-
- Bi L. L., Wang J., Luo Z. Y., Chen S. P., Geng F., Chen Y. H., Li S. J., Yuan C. H., Lin S., Gao T. M., Enhanced excitability in the infralimbic cortex produces anxiety-like behaviors. Neuropharmacology 72, 148–156 (2013). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

