A Translational Regulatory Mechanism Mediated by Hypusinated Eukaryotic Initiation Factor 5A Facilitates β-Cell Identity and Function
- PMID: 38055903
- PMCID: PMC10882153
- DOI: 10.2337/db23-0148
A Translational Regulatory Mechanism Mediated by Hypusinated Eukaryotic Initiation Factor 5A Facilitates β-Cell Identity and Function
Abstract
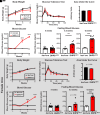
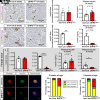
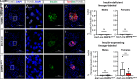
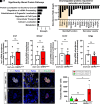
As professional secretory cells, β-cells require adaptable mRNA translation to facilitate a rapid synthesis of proteins, including insulin, in response to changing metabolic cues. Specialized mRNA translation programs are essential drivers of cellular development and differentiation. However, in the pancreatic β-cell, the majority of factors identified to promote growth and development function primarily at the level of transcription. Therefore, despite its importance, the regulatory role of mRNA translation in the formation and maintenance of functional β-cells is not well defined. In this study, we have identified a translational regulatory mechanism mediated by the specialized mRNA translation factor eukaryotic initiation factor 5A (eIF5A), which facilitates the maintenance of β-cell identity and function. The mRNA translation function of eIF5A is only active when it is posttranslationally modified ("hypusinated") by the enzyme deoxyhypusine synthase (DHPS). We have discovered that the absence of β-cell DHPS in mice reduces the synthesis of proteins critical to β-cell identity and function at the stage of β-cell maturation, leading to a rapid and reproducible onset of diabetes. Therefore, our work has revealed a gatekeeper of specialized mRNA translation that permits the β-cell, a metabolically responsive secretory cell, to maintain the integrity of protein synthesis necessary during times of induced or increased demand.
© 2024 by the American Diabetes Association.
Conflict of interest statement
Figures


Similar articles
-
Deoxyhypusine synthase is required for the translational regulation of pancreatic beta cell maturation.bioRxiv [Preprint]. 2023 Apr 24:2023.04.24.537996. doi: 10.1101/2023.04.24.537996. bioRxiv. 2023. PMID: 37162889 Free PMC article. Preprint.
-
Deoxyhypusine synthase, an essential enzyme for hypusine biosynthesis, is required for proper exocrine pancreas development.FASEB J. 2021 May;35(5):e21473. doi: 10.1096/fj.201903177R. FASEB J. 2021. PMID: 33811703 Free PMC article.
-
Deoxyhypusine synthase mutations alter the post-translational modification of eukaryotic initiation factor 5A resulting in impaired human and mouse neural homeostasis.HGG Adv. 2023 May 18;4(3):100206. doi: 10.1016/j.xhgg.2023.100206. eCollection 2023 Jul 13. HGG Adv. 2023. PMID: 37333770 Free PMC article.
-
Post-translational formation of hypusine in eIF5A: implications in human neurodevelopment.Amino Acids. 2022 Apr;54(4):485-499. doi: 10.1007/s00726-021-03023-6. Epub 2021 Jul 17. Amino Acids. 2022. PMID: 34273022 Free PMC article. Review.
-
Hypusine: its post-translational formation in eukaryotic initiation factor 5A and its potential role in cellular regulation.Biofactors. 1993 May;4(2):95-104. Biofactors. 1993. PMID: 8347280 Review.
Cited by
-
Targeting β-Cell Plasticity: A Promising Approach for Diabetes Treatment.Curr Issues Mol Biol. 2024 Jul 18;46(7):7621-7667. doi: 10.3390/cimb46070453. Curr Issues Mol Biol. 2024. PMID: 39057094 Free PMC article. Review.
-
Hypusinated and unhypusinated isoforms of the translation factor eIF5A exert distinct effects in models of pancreas development and function.J Biol Chem. 2025 Feb;301(2):108209. doi: 10.1016/j.jbc.2025.108209. Epub 2025 Jan 18. J Biol Chem. 2025. PMID: 39832654 Free PMC article.
-
Deoxyhypusine synthase deficiency syndrome zebrafish model: aberrant morphology, epileptiform activity, and reduced arborization of inhibitory interneurons.Mol Brain. 2024 Sep 27;17(1):68. doi: 10.1186/s13041-024-01139-w. Mol Brain. 2024. PMID: 39334388 Free PMC article.
References
-
- MacDonald PE, Rorsman P.. The ins and outs of secretion from pancreatic β-cells: control of single-vesicle exo- and endocytosis. Physiology (Bethesda) 2007;22:113–121 - PubMed
-
- Parreiras-e-Silva LT, Luchessi AD, Reis RI, et al. . Evidences of a role for eukaryotic translation initiation factor 5A (eIF5A) in mouse embryogenesis and cell differentiation. J Cell Physiol 2010;225:500–505 - PubMed
-
- Pan FC, Wright C.. Pancreas organogenesis: from bud to plexus to gland. Dev Dyn 2011;240:530–565 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

