An excitatory projection from the basal forebrain to the ventral tegmental area that underlies anorexia-like phenotypes
- PMID: 38056455
- PMCID: PMC10922337
- DOI: 10.1016/j.neuron.2023.11.001
An excitatory projection from the basal forebrain to the ventral tegmental area that underlies anorexia-like phenotypes
Abstract
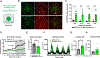
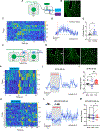
Maladaptation in balancing internal energy needs and external threat cues may result in eating disorders. However, brain mechanisms underlying such maladaptations remain elusive. Here, we identified that the basal forebrain (BF) sends glutamatergic projections to glutamatergic neurons in the ventral tegmental area (VTA) in mice. Glutamatergic neurons in both regions displayed correlated responses to various stressors. Notably, in vivo manipulation of BF terminals in the VTA revealed that the glutamatergic BF → VTA circuit reduces appetite, increases locomotion, and elicits avoidance. Consistently, activation of VTA glutamatergic neurons reduced body weight, blunted food motivation, and caused hyperactivity with behavioral signs of anxiety, all hallmarks of typical anorexia symptoms. Importantly, activation of BF glutamatergic terminals in the VTA reduced dopamine release in the nucleus accumbens. Collectively, our results point to overactivation of the glutamatergic BF → VTA circuit as a potential cause of anorexia-like phenotypes involving reduced dopamine release.
Keywords: VTA; anorexia; anxiety; basal forebrain; dopamine; feeding; glutamatergic neurons; hyperactivity; stress; voluntary hypophagia.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

