Tubular mitochondrial pyruvate carrier disruption elicits redox adaptations that protect from acute kidney injury
- PMID: 38056691
- PMCID: PMC10733108
- DOI: 10.1016/j.molmet.2023.101849
Tubular mitochondrial pyruvate carrier disruption elicits redox adaptations that protect from acute kidney injury
Abstract
Objective: Energy-intensive kidney reabsorption processes essential for normal whole-body function are maintained by tubular epithelial cell metabolism. Although tubular metabolism changes markedly following acute kidney injury (AKI), it remains unclear which metabolic alterations are beneficial or detrimental. By analyzing large-scale, publicly available datasets, we observed that AKI consistently leads to downregulation of the mitochondrial pyruvate carrier (MPC). This investigation aimed to understand the contribution of the tubular MPC to kidney function, metabolism, and acute injury severity.
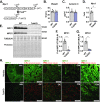
Methods: We generated tubular epithelial cell-specific Mpc1 knockout (MPC TubKO) mice and employed renal function tests, in vivo renal 13C-glucose tracing, mechanistic enzyme activity assays, and tests of injury and survival in an established rhabdomyolysis model of AKI.
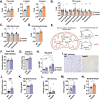
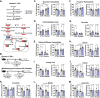
Results: MPC TubKO mice retained normal kidney function, displayed unchanged markers of kidney injury, but exhibited coordinately increased enzyme activities of the pentose phosphate pathway and the glutathione and thioredoxin oxidant defense systems. Following rhabdomyolysis-induced AKI, compared to WT control mice, MPC TubKO mice showed increased glycolysis, decreased kidney injury and oxidative stress markers, and strikingly increased survival.
Conclusions: Our findings suggest that decreased renal tubular mitochondrial pyruvate uptake hormetically upregulates oxidant defense systems before AKI and is a beneficial adaptive response after rhabdomyolysis-induced AKI. This raises the possibility of therapeutically modulating the MPC to attenuate AKI severity.
Keywords: Acute kidney injury; Hormesis; Metabolomics; Mitochondrial metabolism; Oxidative stress.
Copyright © 2023 The Author(s). Published by Elsevier GmbH.. All rights reserved.
Figures




Update of
-
Tubular Mitochondrial Pyruvate Carrier Disruption Elicits Redox Adaptations that Protect from Acute Kidney Injury.bioRxiv [Preprint]. 2023 Jan 31:2023.01.31.526492. doi: 10.1101/2023.01.31.526492. bioRxiv. 2023. Update in: Mol Metab. 2024 Jan;79:101849. doi: 10.1016/j.molmet.2023.101849. PMID: 36778297 Free PMC article. Updated. Preprint.
References
-
- Chawla L.S., Bellomo R., Bihorac A., Goldstein S.L., Siew E.D., Bagshaw S.M., et al. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol. 2017;13(4):241–257. - PubMed
-
- Chertow G.M., Burdick E., Honour M., Bonventre J.V., Bates D.W. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16(11):3365–3370. - PubMed
-
- Goldstein S.L., Jaber B.L., Faubel S., Chawla L.S. AKI transition of care: a potential opportunity to detect and prevent CKD. Clin J Am Soc Nephrol. 2013;8(3):476–483. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

