Cooperative actin filament nucleation by the Arp2/3 complex and formins maintains the homeostatic cortical array in Arabidopsis epidermal cells
- PMID: 38057163
- PMCID: PMC10896301
- DOI: 10.1093/plcell/koad301
Cooperative actin filament nucleation by the Arp2/3 complex and formins maintains the homeostatic cortical array in Arabidopsis epidermal cells
Abstract
Precise control over how and where actin filaments are created leads to the construction of unique cytoskeletal arrays within a common cytoplasm. Actin filament nucleators are key players in this activity and include the conserved actin-related protein 2/3 (Arp2/3) complex as well as a large family of formins. In some eukaryotic cells, these nucleators compete for a common pool of actin monomers and loss of one favors the activity of the other. To test whether this mechanism is conserved, we combined the ability to image single filament dynamics in the homeostatic cortical actin array of living Arabidopsis (Arabidopsis thaliana) epidermal cells with genetic and/or small molecule inhibitor approaches to stably or acutely disrupt nucleator activity. We found that Arp2/3 mutants or acute CK-666 treatment markedly reduced the frequency of side-branched nucleation events as well as overall actin filament abundance. We also confirmed that plant formins contribute to side-branched filament nucleation in vivo. Surprisingly, simultaneous inhibition of both classes of nucleator increased overall actin filament abundance and enhanced the frequency of de novo nucleation events by an unknown mechanism. Collectively, our findings suggest that multiple actin nucleation mechanisms cooperate to generate and maintain the homeostatic cortical array of plant epidermal cells.
© The Author(s) 2023. Published by Oxford University Press on behalf of American Society of Plant Biologists.
Conflict of interest statement
Conflict of interest statement. None declared.
Figures

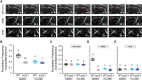
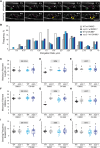
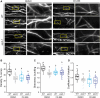



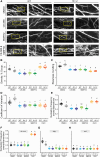

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

