Docking for EP4R antagonists active against inflammatory pain
- PMID: 38057319
- PMCID: PMC10700596
- DOI: 10.1038/s41467-023-43506-6
Docking for EP4R antagonists active against inflammatory pain
Abstract
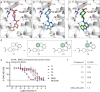
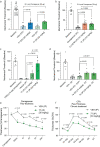
The lipid prostaglandin E2 (PGE2) mediates inflammatory pain by activating G protein-coupled receptors, including the prostaglandin E2 receptor 4 (EP4R). Nonsteroidal anti-inflammatory drugs (NSAIDs) reduce nociception by inhibiting prostaglandin synthesis, however, the disruption of upstream prostanoid biosynthesis can lead to pleiotropic effects including gastrointestinal bleeding and cardiac complications. In contrast, by acting downstream, EP4R antagonists may act specifically as anti-inflammatory agents and, to date, no selective EP4R antagonists have been approved for human use. In this work, seeking to diversify EP4R antagonist scaffolds, we computationally dock over 400 million compounds against an EP4R crystal structure and experimentally validate 71 highly ranked, de novo synthesized molecules. Further, we show how structure-based optimization of initial docking hits identifies a potent and selective antagonist with 16 nanomolar potency. Finally, we demonstrate favorable pharmacokinetics for the discovered compound as well as anti-allodynic and anti-inflammatory activity in several preclinical pain models in mice.
© 2023. The Author(s).
Conflict of interest statement
B.K.S. is founder of Epiodyne, BlueDolphin, and Deep Apple Therapeutics, serves on SABs for Schrodinger LLC, Umbra Therapeutics, Vilya Therapeutics, and consults for Great Point Ventures and for Levator Therapeutics. J.J.I. is founder of BlueDolphin and Deep Apple Therapeutics. Y.S.M. is CEO of Chemspace LLC and scientific advisor at Enamine, Ltd. B.L.R. is founder of Onsero Therapeutics. S.G. and E.A.F. are employed at Deep Apple Therapeutics. The remaining authors declare no competing interest.
Figures

References
-
- Chen, L., Yang, G. & Grosser, T. Prostanoids and inflammatory pain. in Prostaglandins and Other Lipid Mediators 104–105 58–66 (Elsevier, 2013). - PubMed
-
- Penning TD, et al. Synthesis and Biological Evaluation of the 1,5-Diarylpyrazole Class of Cyclooxygenase-2 Inhibitors: Identification of 4-[5-(4-Methylphenyl)−3- (trifluoromethyl)−1H-pyrazol-1-yl]benzenesulfonamide (SC-58635, Celecoxib.). J. Med. Chem. 1997;40:1347–1365. doi: 10.1021/jm960803q. - DOI - PubMed

