In operando NMR investigations of the aqueous electrolyte chemistry during electrolytic CO2 reduction
- PMID: 38057421
- PMCID: PMC10700511
- DOI: 10.1038/s42004-023-01065-3
In operando NMR investigations of the aqueous electrolyte chemistry during electrolytic CO2 reduction
Erratum in
-
Author Correction: In operando NMR investigations of the aqueous electrolyte chemistry during electrolytic CO2 reduction.Commun Chem. 2024 Apr 1;7(1):72. doi: 10.1038/s42004-024-01156-9. Commun Chem. 2024. PMID: 38561501 Free PMC article. No abstract available.
Abstract
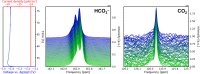
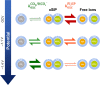
The electrolytic reduction of CO2 in aqueous media promises a pathway for the utilization of the green house gas by converting it to base chemicals or building blocks thereof. However, the technology is currently not economically feasible, where one reason lies in insufficient reaction rates and selectivities. Current research of CO2 electrolysis is becoming aware of the importance of the local environment and reactions at the electrodes and their proximity, which can be only assessed under true catalytic conditions, i.e. by in operando techniques. In this work, multinuclear in operando NMR techniques were applied in order to investigate the evolution of the electrolyte chemistry during CO2 electrolysis. The CO2 electroreduction was performed in aqueous NaHCO3 or KHCO3 electrolytes at silver electrodes. Based on 13C and 23Na NMR studies at different magnetic fields, it was found that the dynamic equilibrium of the electrolyte salt in solution, existing as ion pairs and free ions, decelerates with increasingly negative potential. In turn, this equilibrium affects the resupply rate of CO2 to the electrolysis reaction from the electrolyte. Substantiated by relaxation measurements, a mechanism was proposed where stable ion pairs in solution catalyze the bicarbonate dehydration reaction, which may provide a new pathway for improving educt resupply during CO2 electrolysis.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests
Figures







References
-
- Fenton HJH. LXIV.–the reduction of carbon dioxide to formaldehyde in aqueous solution. J. Chem. Soc. Trans. 1907;91:687–693. doi: 10.1039/CT9079100687. - DOI
-
- Whipple DT, Kenis PJA. Prospects of CO2 utilization via direct heterogeneous electrochemical reduction. J. Phys. Chem. Lett. 2010;1:3451–3458. doi: 10.1021/jz1012627. - DOI
-
- Jhong HR, Ma SC, Kenis PJA. Electrochemical conversion of CO2 to useful chemicals: current status, remaining challenges, and future opportunities. Curr. Opin. Chem. Eng. 2013;2:191–199. doi: 10.1016/j.coche.2013.03.005. - DOI
-
- Jeanty P, et al. Upscaling and continuous operation of electrochemical CO2 to CO conversion in aqueous solutions on silver gas diffusion electrodes. J. CO. 2018;24:454–462.
Grants and funding
LinkOut - more resources
Full Text Sources

