SMAD4 endows TGF-β1-induced highly invasive tumor cells with ferroptosis vulnerability in pancreatic cancer
- PMID: 38057506
- PMCID: PMC10943101
- DOI: 10.1038/s41401-023-01199-z
SMAD4 endows TGF-β1-induced highly invasive tumor cells with ferroptosis vulnerability in pancreatic cancer
Abstract
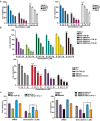
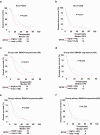
Pancreatic ductal adenocarcinoma (PDAC) is an extremely aggressive malignancy prone to recurrence and metastasis. Studies show that tumor cells with increased invasive and metastatic potential are more likely to undergo ferroptosis. SMAD4 is a critical molecule in the transforming growth factor β (TGF-β) pathway, which affects the TGF-β-induced epithelial-mesenchymal transition (EMT) status. SMAD4 loss is observed in more than half of patients with PDAC. In this study, we investigated whether SMAD4-positive PDAC cells were prone to ferroptosis because of their high invasiveness. We showed that SMAD4 status almost determined the orientation of transforming growth factor β1 (TGF-β1)-induced EMT via the SMAD4-dependent canonical pathway in PDAC, which altered ferroptosis vulnerability. We identified glutathione peroxidase 4 (GPX4), which inhibited ferroptosis, as a SMAD4 down-regulated gene by RNA sequencing. We found that SMAD4 bound to the promoter of GPX4 and decreased GPX4 transcription in PDAC. Furthermore, TGF-β1-induced high invasiveness enhanced sensitivity of SMAD4-positive organoids and pancreas xenograft models to the ferroptosis inducer RAS-selective lethal 3 (RSL3). Moreover, SMAD4 enhanced the cytotoxic effect of gemcitabine combined with RSL3 in highly invasive PDAC cells. This study provides new ideas for the treatment of PDAC, especially SMAD4-positive PDAC.
Keywords: EMT; GPX4; RSL3; SMAD4; ferroptosis; pancreatic cancer.
© 2023. The Author(s), under exclusive licence to Shanghai Institute of Materia Medica, Chinese Academy of Sciences and Chinese Pharmacological Society.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- GBD 2017 Pancreatic Cancer Collaborators. The global, regional, and national burden of pancreatic cancer and its attributable risk factors in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2019;4:934–47. doi: 10.1016/S2468-1253(19)30347-4. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

