Filgotinib Demonstrates Efficacy in Rheumatoid Arthritis Independent of Smoking Status: Post Hoc Analysis of Phase 3 Trials and Claims-Based Analysis
- PMID: 38057656
- PMCID: PMC10796882
- DOI: 10.1007/s40744-023-00619-0
Filgotinib Demonstrates Efficacy in Rheumatoid Arthritis Independent of Smoking Status: Post Hoc Analysis of Phase 3 Trials and Claims-Based Analysis
Abstract
Objectives: To assess cigarette smoking's effects on efficacy of the preferential Janus kinase (JAK) 1 inhibitor filgotinib and drug persistence in patients with rheumatoid arthritis (RA).
Methods: Efficacy in non-smokers, former smokers, and current smokers from phase 3 filgotinib trials was analyzed, including patients with inadequate response (IR) to methotrexate (MTX) or biologic disease-modifying antirheumatic drugs (bDMARDs) or who were MTX-naïve. Proportions achieving Disease Activity Score in 28 joints with C-reactive protein (DAS28[CRP]) ≤ 3.2 were compared using logistic regression. Retrospective claims-based switching data were reviewed.
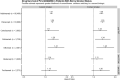
Results: Week 12 (W12) DAS28(CRP) ≤ 3.2 was achieved by 50, 61, and 62% of MTX-IR non-smokers, former smokers, and current smokers taking filgotinib 200 mg (FIL200) + MTX vs. 23, 16, and 32% taking placebo + MTX (p < 0.001, < 0.001, and 0.001) and 50, 34, and 33% taking adalimumab + MTX (p = 0.97, 0.013, and 0.006 vs. FIL200 + MTX). W12 DAS28(CRP) ≤ 3.2 was achieved by 46, 48, and 32% of bDMARD-IR non-smokers, former smokers, and current smokers taking FIL200 + conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) vs. 16, 23, and 5% taking placebo + csDMARD (p < 0.001, 0.077, and 0.051); 57, 58, and 59% of respective MTX-naïve smoking groups achieved W12 DAS28(CRP) ≤ 3.2 with FIL200 + MTX vs. 28, 37, and 18% with MTX (p < 0.001, 0.026, and < 0.001). Claims data showed former/current smokers were likelier than non-smokers to switch from adalimumab to other biologics or JAK inhibitors.
Conclusions: Greater proportions of MTX-IR current/former smokers responded to FIL200 + MTX vs. adalimumab + MTX. In non-smoking MTX-IR, bDMARD-IR, and MTX-naïve patients with RA, FIL200 + MTX demonstrated increased response vs. controls. Current/former smokers were likelier to discontinue adalimumab vs. non-smokers in real-world clinical settings.
Trial registration: NCT02889796, NCT02873936, NCT02886728.
Keywords: Arthritis; JAK inhibitors; Rheumatoid; Smoking; Tumor necrosis factor inhibitors.
© 2023. The Author(s).
Conflict of interest statement
Jeffrey R. Curtis has served as a consultant for AbbVie, Amgen, Bendcare, Bristol Myers Squibb, Corrona, Eli Lilly, Janssen, Myriad, Novartis, Pfizer, Regeneron, Roche, and UCB; and has received grant/research support from AbbVie, Amgen, Bristol Myers Squibb, Corrona, Eli Lilly, Janssen, Myriad, Pfizer, Regeneron, Roche, and UCB. Paul Emery receives grant/research support from AbbVie, Bristol Myers Squibb, Eli Lilly, and Samsung; and is a consultant for AbbVie; Bristol Myers Squibb; Celltrion; Gilead Sciences, Inc.; Eli Lilly; Novartis; Roche; Samsung; and Sandoz. Bryan Downie, Yan Zhong, and Ling Han are shareholders and employees of Gilead Sciences, Inc. Rachael E. Hawtin is a former employee of Gilead Sciences, Inc., and a current shareholder, and is a current employee of Ultragenyx Pharmaceutical Inc. Jinfeng Liu is a shareholder and employee of Gilead Sciences, Inc., and shareholder of Roche. Gerd Rüdiger Burmester reports serving as a consultant and on a speakers bureau for AbbVie, Eli Lilly, Pfizer, and Gilead Sciences, Inc.
Figures

References
-
- Hyrich KL, Watson KD, Silman AJ, Symmons DP, British Society for Rheumatology Biologics Register Predictors of response to anti-TNF-alpha therapy among patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register. Rheumatology (Oxford) 2006;45(12):1558–1565. doi: 10.1093/rheumatology/kel149. - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

