Incidence and risk factors of hypothyroidism after treatment for early breast cancer: a population-based cohort study
- PMID: 38057688
- PMCID: PMC10805818
- DOI: 10.1007/s10549-023-07184-8
Incidence and risk factors of hypothyroidism after treatment for early breast cancer: a population-based cohort study
Abstract
Purpose: An increased incidence of hypothyroidism among breast cancer survivors has been observed in earlier studies. The impact of the postoperative treatment modalities and their potential interplay on hypothyroidism development needs to be studied.
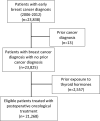
Methods: We conducted a population- and registry-based study using the Breast Cancer Data Base Sweden (BCBaSe) including females diagnosed with breast cancer between 2006 and 2012. In total, 21,268 female patients diagnosed with early breast cancer between 2006 and 2012, with no previous prescription of thyroid hormones and no malignant diagnosis during the last ten years before breast cancer diagnosis, were included in the final analysis.
Results: During the follow-up (median follow-up time 7.9 years), 1212 patients (5.7%) developed hypothyroidism at a median time of 3.45 years from the index date. No association of the systemic oncological treatment in terms of either chemotherapy or endocrine therapy and hypothyroidism development could be identified. A higher risk (HR 1.68;95% CI 1.42-1.99) of hypothyroidism identified among patients treated with radiation treatment of the regional lymph nodes whereas no increased risk in patients treated only with radiation therapy to the breast/chest wall was found (HR 1.01; 95% CI 0.86-1.19). The risk of hypothyroidism in the cohort treated with radiotherapy of the regional lymph nodes was present irrespective of the use of adjuvant chemotherapy treatment.
Conclusions: Based on the results of our study, the implementation of hypothyroidism surveillance among the breast cancer survivors treated with radiotherapy of the regional lymph nodes can be considered as reasonable in the follow-up program.
Keywords: Breast cancer; Chemotherapy; Endocrine therapy; Hypothyroidism; Population-based; Radiation therapy.
© 2023. The Author(s).
Conflict of interest statement
Financial interests: AM has performed consultancy for Veracyte (no financial or other compensation) and Roche (no financial or other compensation). ET has received speaker honoraria from Roche. Non-financial interests: AM has served on advisory boards for Veracyte and Roche, while he has received research funding paid to institution by AstraZeneca and Novartis. Authors ED, DS, AKW, and AV declare they have no conflict of interests.
Figures
References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical