Immunological and biochemical biomarker alterations among SARS-COV-2 patients with varying disease phenotypes in Uganda
- PMID: 38057707
- PMCID: PMC10701962
- DOI: 10.1186/s12879-023-08854-0
Immunological and biochemical biomarker alterations among SARS-COV-2 patients with varying disease phenotypes in Uganda
Abstract
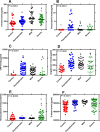
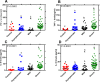
Every novel infection requires an assessment of the host response coupled with identification of unique biomarkers for predicting disease pathogenesis, treatment targets and diagnostic utility. Studies have exposed dysregulated inflammatory response induced by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as significant predictor or cause of disease severity/prognosis and death. This study evaluated inflammatory biomarkers induced by SARS-CoV-2 in plasma of patients with varying disease phenotypes and healthy controls with prognostic or therapeutic potential. We stratified SARS-CoV-2 plasma samples based on disease status (asymptomatic, mild, severe, and healthy controls), as diagnosed by RT-PCR SARS-CoV-2. We used a solid phase sandwich and competitive Enzyme-Linked Immunosorbent Assay (ELISA) to measure levels of panels of immunological (IFN-γ, TNF-α, IL-6, and IL-10) and biochemical markers (Ferritin, Procalcitonin, C-Reactive Protein, Angiotensin II, Homocysteine, and D-dimer). Biomarker levels were compared across SARS-CoV-2 disease stratification. Plasma IFN-γ, TNF-α, IL-6, and IL-10 levels were significantly (P < 0.05) elevated in the severe SARS-CoV-2 patients as compared to mild, asymptomatic, and healthy controls. Ferritin, Homocysteine, and D-dimer plasma levels were significantly elevated in severe cases over asymptomatic and healthy controls. Plasma C-reactive protein and Angiotensin II levels were significantly (P < 0.05) higher in mild than severe cases and healthy controls. Plasma Procalcitonin levels were significantly higher in asymptomatic than in mild, severe cases and healthy controls. Our study demonstrates the role of host inflammatory biomarkers in modulating the pathogenesis of COVID-19. The study proposes a number of potential biomarkers that could be explored as SARS-CoV-2 treatment targets and possible prognostic predictors for a severe outcome. The comprehensive analysis of prognostic biomarkers may contribute to the evidence-based management of COVID-19 patients.
Keywords: Biomarkers; COVID-19; Cytokine; Inflammation; SARS-CoV-2.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- WHO., “Coronavirus disease (COVID-19),” no. September, 2020.
-
- Saksena N, Bonam SR, Miranda-Saksena M. Immunopathogenesis of severe acute respiratory syndrome coronavirus-2: evolving knowledge and its current status. Explor Immunol. 2021;1:61–79. doi: 10.37349/ei.2021.00007. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

