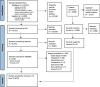
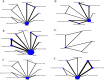
Clinical effectiveness of pharmacological interventions for managing chronic migraine in adults: a systematic review and network meta-analysis
- PMID: 38057728
- PMCID: PMC10702068
- DOI: 10.1186/s10194-023-01696-w
Clinical effectiveness of pharmacological interventions for managing chronic migraine in adults: a systematic review and network meta-analysis
Abstract
Background: Chronic migraine can be a profoundly disabling disorder that may be treated with preventive medications. However, uncertainty remains as to which preventive medication is the most effective. We present a network meta-analysis to determine the effectiveness and rank of preventive drugs for chronic migraine in adults.
Methods: We identified, reviewed, and extracted data from randomised controlled trials (RCTs) of preventive drugs for chronic migraine with at least 200 participants. Data were analysed using network meta-analysis.
Findings: We included 12 RCTs of six medications (Eptinezumab, Erenumab, Fremanezumab, Galcanezumab, Onabotulinumtoxin A, and Topiramate) compared to placebo or each other. All drugs effectively reduced monthly headache and migraine days compared with placebo. The most effective drug for monthly headache days was Eptinezumab 300mg, with a mean difference of -2.46 days, 95% Credible Interval (CrI): -3.23 to -1.69. On the Surface Under the Cumulative Ranking Area (SUCRA) analysis, the probability that Eptinezumab 300mg was ranked highest was 0.82. For monthly migraine days, the most effective medication was Fremanezumab-monthly, with a mean difference: -2.77 days, 95% CrI: -3.36 to -2.17, and 0.98 probability of being ranked the highest. All included drugs, except Topiramate, improved headache-related quality of life. No eligible studies were identified for the other common preventive oral medications such as Amitriptyline, Candesartan, and Propranolol. The main reasons were that the studies did not define chronic migraine, were undertaken before the definition of chronic migraine, or were too small.
Interpretation: All six medications were more effective than the placebo on monthly headache and migraine days. The absolute differences in the number of headache/migraine days are, at best, modest. No evidence was found to determine the relative effectiveness of the six included drugs with other oral preventive medications.
Registration: PROSPERO (number CRD42021265990).
© 2023. The Author(s).
Conflict of interest statement
Martin Underwood is chief investigator or co-investigator on multiple previous and current research grants from the UK National Institute for Health Research and is a co-investigator on grants funded by the Australian NHMRC and Norwegian MRC. He was an NIHR Senior Investigator until March 2021. He is a director and shareholder of Clinvivo Ltd, which provides electronic data collection for health services research. He is part of an academic partnership with Serco Ltd, funded by the European Social Fund, related to return-to-work initiatives. He receives some salary support from University Hospitals Coventry and Warwickshire. He is a co-investigator on two current and one completed NIHR-funded studies that have, or have had, additional support from Stryker Ltd.
Amy Grove is a member of the NIHR HTA Commissioning Committee and a member of the NIHR DSE Fellowship Funding Committee.
Callum Duncan is chair of Scottish Intercollegiate Guideline Network (SIGN) 155 and has provided advice on the use of Botox, CGRP monoclonal antibodies and CGRP antagonists to the Scottish Medicines Consortium and on Eptinezumab to NICE. He was the Secretary for the British Association for the Study of Headache 2015–2022 and is a Board member of Anglo Dutch Migraine Association.
Manjit Matharu is the President of the medical advisory board of the CSF Leak Association. He has received consulting fees from AbbVie, TEVA, Lundbeck, Eli Lilly, Salvia, and Pfizer. He has received payment for the development of educational presentations from AbbVie, Pfizer and Eli Lilly and support for attending a meeting from Pfizer. He is on the advisory board for AbbVie, TEVA, Lunbeck, Eli Lilly, Salvia and Pfizer. He has the following patent issued WO2018051103A1: System and method for diagnosing and treating headaches. He has stock options with Tesla, Adobe, Nvidia, META, and Microsoft. He has received grants from Abbott, Medtronic and Ehlers Danlos society.
Hema Mistry is a member of the NIHR HTA General Funding Commissioning Committee.
Figures
References
-
- Lantéri-Minet M, Duru G, Mudge M, et al. Quality of life impairment, disability and economic burden associated with chronic daily headache, focusing on chronic migraine with or without medication overuse: a systematic review. Cephalalgia. 2011;31(7):837–850. doi: 10.1177/0333102411398400. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical