Phase I dose escalation study and pilot efficacy analysis of LXI-15029, a novel mTOR dual inhibitor, in Chinese subjects with advanced malignant solid tumors
- PMID: 38057772
- PMCID: PMC10702058
- DOI: 10.1186/s12885-023-11578-8
Phase I dose escalation study and pilot efficacy analysis of LXI-15029, a novel mTOR dual inhibitor, in Chinese subjects with advanced malignant solid tumors
Abstract
Background: The mammalian target of rapamycin (mTOR) kinase, a central component of the PI3K/AKT/mTOR pathway, plays a critical role in tumor biology as an attractive therapeutic target. We conducted this first-in-human study to investigate the safety, pharmacokinetics (PK), and pilot efficacy of LXI-15029, an mTORC1/2 dual inhibitor, in Chinese patients with advanced malignant solid tumors.
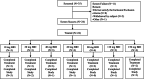
Methods: Eligible patients with advanced, unresectable malignant solid tumors after failure of routine therapy or with no standard treatment were enrolled to receive ascending doses (10, 20, 40, 60, 80, 110, and 150 mg) of oral LXI-15029 twice daily (BID) (3 + 3 dose-escalation pattern) until disease progression or intolerable adverse events (AEs). The primary endpoints were safety and tolerability.
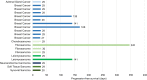
Results: Between June 2017 and July 2021, a total of 24 patients were enrolled. LXI-15029 was well tolerated at all doses. Only one dose-limiting toxicity (grade 3 increased alanine aminotransferase) occurred in the 150 mg group, and the maximum tolerated dose was 110 mg BID. The most common treatment-related AEs were leukocytopenia (41.7%), increased alanine aminotransferase (20.8%), increased aspartate aminotransferase (20.8%), prolonged electrocardiogram QT interval (20.8%), and hypertriglyceridemia (20.8%). No other serious treatment-related AEs were reported. LXI-15029 was absorbed rapidly after oral administration. The increases in the peak concentration and the area under the curve were greater than dose proportionality over the dose range. Eight patients had stable disease. The disease control rate was 40.0% (8/20; 95% CI 21.7-60.6). In evaluable patients, the median progression-free survival was 29 days (range 29-141).
Conclusions: LXI-15029 demonstrated reasonable safety and tolerability profiles and encouraging preliminary antitumor activity in Chinese patients with advanced malignant solid tumors, which warranted further validation in phase II trials.
Trial registration: NCT03125746(24/04/2017), http://ClinicalTrials.gov/show/NCT03125746.
Keywords: First-in-human trial; LXI-15029; Metastatic solid tumors; mTORC1/2 dual inhibitor.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous