An engineered Fc fusion protein that targets antigen-specific T cells and autoantibodies mitigates autoimmune disease
- PMID: 38057803
- PMCID: PMC10702099
- DOI: 10.1186/s12974-023-02974-9
An engineered Fc fusion protein that targets antigen-specific T cells and autoantibodies mitigates autoimmune disease
Abstract
Current effective therapies for autoimmune diseases rely on systemic immunomodulation that broadly affects all T and/or B cell responses. An ideal therapeutic approach would combine autoantigen-specific targeting of both T and B cell effector functions, including efficient removal of pathogenic autoantibodies. Albeit multiple strategies to induce T cell tolerance in an autoantigen-specific manner have been proposed, therapeutic removal of autoantibodies remains a significant challenge. Here, we devised an approach to target both autoantigen-specific T cells and autoantibodies by producing a central nervous system (CNS) autoantigen myelin oligodendrocyte glycoprotein (MOG)-Fc fusion protein. We demonstrate that MOG-Fc fusion protein has significantly higher bioavailability than monomeric MOG and is efficient in clearing anti-MOG autoantibodies from circulation. We also show that MOG-Fc promotes T cell tolerance and protects mice from MOG-induced autoimmune encephalomyelitis. This multipronged targeting approach may be therapeutically advantageous in the treatment of autoimmunity.
Keywords: Autoantibodies; EAE; Fc fusion; Multiple sclerosis; Myelin oligodendrocyte glycoprotein (MOG); T cells; Tolerance.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


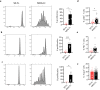
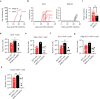
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

