Differences in venous clot structures between hemophilic mice treated with emicizumab versus factor VIII or factor VIIIFc
- PMID: 38058210
- PMCID: PMC11141652
- DOI: 10.3324/haematol.2023.284142
Differences in venous clot structures between hemophilic mice treated with emicizumab versus factor VIII or factor VIIIFc
Abstract
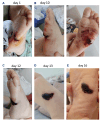
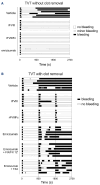
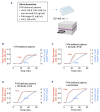
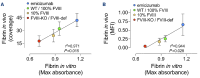
Recombinant factor VIII (rFVIII), rFVIIIFc and emicizumab are established treatment options in the management of hemophilia A. Each has its unique mode of action, which can influence thrombin generation kinetics and therefore also the kinetics of thrombin substrates. Such differences may potentially result in clots with different structural and physical properties. A starting observation of incomplete wound closure in a patient on emicizumab prophylaxis led us to employ a relevant mouse model in which we noticed that emicizumab-induced clots appeared less stable compared to FVIII-induced clots. We therefore analyzed fibrin formation in vitro and in vivo. In vitro fibrin formation was faster and more abundant in the presence of emicizumab than in the presence of rFVIII/rFVIIIFc. Furthermore, the time-interval between the initiation of fibrin formation and factor XIII activation was twice as long for emicizumab than as for rFVIII/rFVIIIFc. Scanning electron microscopy and immunofluorescent spinning-disk confocal microscopy of in vivo-generated clots confirmed increased fibrin formation in the presence of emicizumab. Unexpectedly, we also detected a different morphology between rFVIII/rFVIIIFcand emicizumab-induced clots. Contrary to the regular fibrin mesh obtained with rFVIII/rFVIIIFc, fibrin fibers appeared to be fused into large patches upon emicizumab treatment. Moreover, fewer red blood cells were detected in regions in which these fibrin patches were present. The presence of highly dense fibrin structures associated with a diffuse fiber structure in emicizumab-induced clots was also observed when using super-resolution imaging. We hypothesize that the modified kinetics of thrombin, fibrin and factor XIIIa generation contribute to differences in structural and physical properties between clots formed in the presence of FVIII or emicizumab.
Figures



References
-
- Versteeg HH, Heemskerk JW, Levi M, Reitsma PH. New fundamentals in hemostasis. Physiol Rev. 2013;93(1):327-358. - PubMed
-
- Domingues MM, Macrae FL, Duval C, et al. . Thrombin and fibrinogen gamma’ impact clot structure by marked effects on intrafibrillar structure and protofibril packing. Blood. 2016;127(4):487-495. - PubMed
-
- Lenting PJ, van Mourik JA, Mertens K. The life cycle of coagulation factor VIII in view of its structure and function. Blood. 1998;92(11):3983-3996. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

