Next-Generation Vitrimers Design through Theoretical Understanding and Computational Simulations
- PMID: 38058273
- PMCID: PMC10837359
- DOI: 10.1002/advs.202302816
Next-Generation Vitrimers Design through Theoretical Understanding and Computational Simulations
Abstract
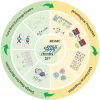
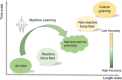
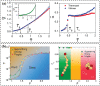
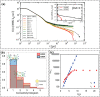
Vitrimers are an innovative class of polymers that boast a remarkable fusion of mechanical and dynamic features, complemented by the added benefit of end-of-life recyclability. This extraordinary blend of properties makes them highly attractive for a variety of applications, such as the automotive sector, soft robotics, and the aerospace industry. At their core, vitrimer materials consist of crosslinked covalent networks that have the ability to dynamically reorganize in response to external factors, including temperature changes, pressure variations, or shifts in pH levels. In this review, the aim is to delve into the latest advancements in the theoretical understanding and computational design of vitrimers. The review begins by offering an overview of the fundamental principles that underlie the behavior of these materials, encompassing their structures, dynamic behavior, and reaction mechanisms. Subsequently, recent progress in the computational design of vitrimers is explored, with a focus on the employment of molecular dynamics (MD)/Monte Carlo (MC) simulations and density functional theory (DFT) calculations. Last, the existing challenges and prospective directions for this field are critically analyzed, emphasizing the necessity for additional theoretical and computational advancements, coupled with experimental validation.
Keywords: Monte Carlo simulations; bond exchange reactions; density functional theory; molecular dynamics simulations; vitrimers.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- a) Zheng J., Png Z. M., Ng S. H., Tham G. X., Ye E., Goh S. S., Loh X. J., Li Z., Mater. Today 2021, 51, 586;
- b) Kloxin C. J., Bowman C. N., Chem. Soc. Rev. 2013, 42, 7161; - PubMed
- c) Kloxin C. J., Scott T. F., Adzima B. J., Bowman C. N., Macromolecules 2010, 43, 2643; - PMC - PubMed
- d) Bowman C., Du Prez F., Kalow J., Polym. Chem. 2020, 11, 5295;
- e) Christensen P. R., Scheuermann A. M., Loeffler K. E., Helms B. A., Nat. Chem. 2019, 11, 442; - PubMed
- f) García J. M., Jones G. O., Virwani K., Mccloskey B. D., Boday D. J., Ter Huurne G. M., Horn H. W., Coady D. J., Bintaleb A. M., Alabdulrahman A. M. S., Alsewailem F., Almegren H. A. A., Hedrick J. L., Science 2014, 344, 732; - PubMed
- g) Shieh P., Zhang W., Husted K. E. L., Kristufek S. L., Xiong B., Lundberg D. J., Lem J., Veysset D., Sun Y., Nelson K. A., Plata D. L., Johnson J. A., Nature 2020, 583, 542; - PMC - PubMed
- h) Montarnal D., Capelot M., Tournilhac F., Leibler L., Science 2011, 334, 965; - PubMed
- i) Scott T. F., Schneider A. D., Cook W. D., Bowman C. N., Science 2005, 308, 1615; - PubMed
- j) Billiet S., De Bruycker K., Driessen F., Goossens H., Van Speybroeck V., Winne J. M., Du Prez F. E., Nat. Chem. 2014, 6, 815. - PubMed
-
- a) Scheutz G. M., Lessard J. J., Sims M. B., Sumerlin B. S., J. Am. Chem. Soc. 2019, 141, 16181; - PubMed
- b) Chakma P., Konkolewicz D., Angew. Chem., Int. Ed. 2019, 58, 9682; - PubMed
- c) Van Zee N. J., Nicolay R., Prog. Polym. Sci. 2020, 104, 101233;
- d) Podgórski M., Fairbanks B. D., Kirkpatrick B. E., Mcbride M., Martinez A., Dobson A., Bongiardina N. J., Bowman C. N., Adv. Mater. 2020, 32, 1906876. - PubMed
-
- a) Wang S., Li B., Zheng J., Surat'man N. E. B., Wu J., Wang N., Xu X., Zhu J., Loh X. J., Li Z., ACS Mater. Lett. 2023, 5, 608;
- b) Png Z. M., Zheng J., Kamarulzaman S., Wang S., Li Z., Goh S. S., Green Chem. 2022, 24, 5978;
- c) Zheng J., Solco S. F. D., Wong C. J. E., Sia S. A., Tan X. Y., Cao J., Yeo J. C. C., Yan W., Zhu Q., Yan Q., Wu J., Suwardi A., Li Z., J. Mater. Chem. A 2022, 10, 19787.
-
- a) Denissen W., Winne J. M., Du Prez F. E., Chem. Sci. 2016, 7, 30; - PMC - PubMed
- b) Zou W., Dong J., Luo Y., Zhao Q., Xie T., Adv. Mater. 2017, 29, 1606100; - PubMed
- c) Zhang Z. P, Rong M. Z., Zhang M. Q., Prog. Polym. Sci. 2018, 80, 39;
- d) Jin Y., Lei Z., Taynton P., Huang S., Zhang W., Matter 2019, 1, 1456;
- e) Guerre M., Taplan C., Winne J. M., Du Prez F. E., Chem. Sci. 2020, 11, 4855; - PMC - PubMed
- f) Zheng N., Xu Y., Zhao Q., Xie T., Chem. Rev. 2021, 121, 1716. - PubMed
