Suppression of lipopolysaccharide-induced COX-2 expression via p38MAPK, JNK, and C/EBPβ phosphorylation inhibition by furomagydarin A, a benzofuran glycoside from Magydaris pastinacea
- PMID: 38058285
- PMCID: PMC11792810
- DOI: 10.1080/14756366.2023.2287420
Suppression of lipopolysaccharide-induced COX-2 expression via p38MAPK, JNK, and C/EBPβ phosphorylation inhibition by furomagydarin A, a benzofuran glycoside from Magydaris pastinacea
Abstract
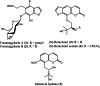
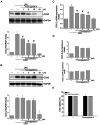
The phytochemical investigation of the methanol extract of the seeds of Magydaris pastinacea afforded two undescribed benzofuran glycosides, furomagydarins A-B (1, 2), together with three known coumarins. The structures of the new isolates were elucidated after extensive 1D and 2D NMR experiments as well as HR MS. Compound 1 was able to inhibit the COX-2 expression in RAW264.7 macrophages exposed to lipopolysaccharide, a pro-inflammatory stimulus. RT-qPCR and luciferase reporter assays suggested that compound 1 reduces COX-2 expression at the transcriptional level. Further studies highlighted the capability of compound 1 to suppress the LPS-induced p38MAPK, JNK, and C/EBPβ phosphorylation, leading to COX-2 down-regulation in RAW264.7 macrophages.
Keywords: C/EBPβ; COX-2; JNK; Magydaris pastinacea; benzofurans; p38MAPK.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures


References
-
- Gorina R, Font-Nieves M, Marquez-Kisinousky L, Santalucia T, Planas AM.. Astrocyte TLR4 activation induces a proinflammatory environment through the interplay between MyD88-dependent NF kappa B signaling, MAPK, and Jak1/Stat1 pathways. Glia. 2011;59(2):242–255. - PubMed
-
- Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F.. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem. 1999;274(16):10689–10692. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
