Improved catalytic performance in gas-phase dimethyl ether carbonylation over facile NH4F etched ferrierite
- PMID: 38058555
- PMCID: PMC10696424
- DOI: 10.1039/d3ra07084k
Improved catalytic performance in gas-phase dimethyl ether carbonylation over facile NH4F etched ferrierite
Abstract
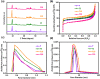
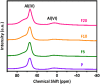
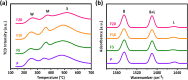
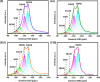
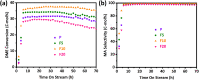
Gas-phase dimethyl ether (DME) carbonylation to methyl acetate (MA) initiates a promising route for producing ethanol from syngas. Ferrierite (FER, ZSM-35) has received considerable attention as it displays excellent stability in the carbonylation reaction and its modification strategy is to improve its catalytic activity on the premise of maintaining its stability as much as possible. However, conventional post-treatment methods such as dealumination and desilication usually selectively remove framework Al or Si atoms, ultimately altering the intrinsic composition, crystallinity, and acidity of zeolites inevitably. In this study, we successfully prepared a series of hierarchical ZSM-35 materials through post-treatment with NH4F etching, which dissolved framework Al and Si at similar rates and preferentially attacked the defective sites. Interestingly, the produced pore systems effectively penetrated the [100] plane, offering elevated access to both the 8-membered ring (8-MR) and 10-membered ring (10-MR) channels. The physicochemical and acid properties of the pristine and NH4F etched ZSM-35 samples were comprehensively characterized using various techniques, including XRD, XRF, FESEM, HRTEM, Nitrogen adsorption-desorption, NH3-TPD, Py-IR, 27Al MAS NMR, and 29Si MAS NMR. Under moderate treatment conditions, the intrinsic microporous structure, acid properties, and crystallinity of zeolite were retained, leading to superior catalytic activity and stability with respect to the pristine sample. Nonetheless, severe NH4F etching disrupted the crystalline framework and created additional defective sites, bringing about faster deposition of coke precursors on the interior Brønsted acid sites (BAS) and decreased catalytic performance. This technique provides a novel and efficient method to slightly enhance the micropore and mesopore volume of industrially pertinent zeolites through a straightforward post-treatment, thus elevating the catalytic performance of these zeolites.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
-
- Spivey J. J. Egbebi A. Chem. Soc. Rev. 2007;36:1514. - PubMed
-
- Yue H. Ma X. Gong J. Acc. Chem. Res. 2014;47:1483–1492. - PubMed
-
- Liu G. Yang G. Peng X. Wu J. Tsubaki N. Chem. Soc. Rev. 2022;51:5606–5659. - PubMed
-
- Göransson K. Söderlind U. He J. Zhang W. Renewable Sustainable Energy Rev. 2011;15:482–492.
-
- Gong J. Yue H. Zhao Y. Zhao S. Zhao L. Lv J. Wang S. Ma X. J. Am. Chem. Soc. 2012;134:13922–13925. - PubMed
LinkOut - more resources
Full Text Sources

