Hypoxia and inflammatory factor preconditioning enhances the immunosuppressive properties of human umbilical cord mesenchymal stem cells
- PMID: 38058960
- PMCID: PMC10696190
- DOI: 10.4252/wjsc.v15.i11.999
Hypoxia and inflammatory factor preconditioning enhances the immunosuppressive properties of human umbilical cord mesenchymal stem cells
Abstract
Background: Mesenchymal stem cells (MSCs) have great potential for the treatment of various immune diseases due to their unique immunomodulatory properties. However, MSCs exposed to the harsh inflammatory environment of damaged tissue after intravenous transplantation cannot exert their biological effects, and therefore, their therapeutic efficacy is reduced. In this challenging context, an in vitro preconditioning method is necessary for the development of MSC-based therapies with increased immunomodulatory capacity and transplantation efficacy.
Aim: To determine whether hypoxia and inflammatory factor preconditioning increases the immunosuppressive properties of MSCs without affecting their biological characteristics.
Methods: Umbilical cord MSCs (UC-MSCs) were pretreated with hypoxia (2% O2) exposure and inflammatory factors (interleukin-1β, tumor necrosis factor-α, interferon-γ) for 24 h. Flow cytometry, polymerase chain reaction, enzyme-linked immunosorbent assay and other experimental methods were used to evaluate the biological characteristics of pretreated UC-MSCs and to determine whether pretreatment affected the immunosuppressive ability of UC-MSCs in coculture with immune cells.
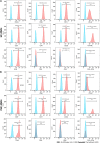
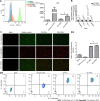
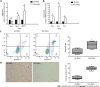
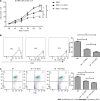
Results: Pretreatment with hypoxia and inflammatory factors caused UC-MSCs to be elongated but did not affect their viability, proliferation or size. In addition, pretreatment significantly decreased the expression of coagulation-related tissue factors but did not affect the expression of other surface markers. Similarly, mitochondrial function and integrity were retained. Although pretreatment promoted UC-MSC apoptosis and senescence, it increased the expression of genes and proteins related to immune regulation. Pretreatment increased peripheral blood mononuclear cell and natural killer (NK) cell proliferation rates and inhibited NK cell-induced toxicity to varying degrees.
Conclusion: In summary, hypoxia and inflammatory factor preconditioning led to higher immunosuppressive effects of MSCs without damaging their biological characteristics.
Keywords: Hypoxia, Inflammatory factors; Immune regulation; Mesenchymal stem cells; Preconditioning; Umbilical cord.
©The Author(s) 2023. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The authors report no relevant conflicts of interest for this article.
Figures


References
-
- Stefańska K, Nemcova L, Blatkiewicz M, Pieńkowski W, Ruciński M, Zabel M, Mozdziak P, Podhorska-Okołów M, Dzięgiel P, Kempisty B. Apoptosis Related Human Wharton's Jelly-Derived Stem Cells Differentiation into Osteoblasts, Chondrocytes, Adipocytes and Neural-like Cells-Complete Transcriptomic Assays. Int J Mol Sci. 2023;24 - PMC - PubMed
LinkOut - more resources
Full Text Sources

