Female pond bats hunt in other areas than males and consume lighter prey when pregnant
- PMID: 38059006
- PMCID: PMC10697422
- DOI: 10.1093/jmammal/gyad096
Female pond bats hunt in other areas than males and consume lighter prey when pregnant
Abstract
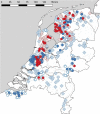
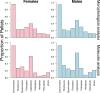
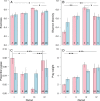
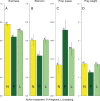
Animals with large energy requirements are forced to optimize their hunting strategy, which may result in differentiation of the diet between sexes and across seasons. Here, we examined spatiotemporal variation in the diet of both sexes of the Pond Bat Myotis dasycneme, a species known to have spatial segregation of sexes when the young are born and lactating. Fecal pellets were collected from live animals for a period of 15 years at various locations in the Netherlands. A total of 535 pellets were successfully analyzed by microscopy and an additional 160 pellets by DNA metabarcoding. Morphological and molecular analyses showed that the diet of pregnant and lactating pond bats differed significantly from the diet of females with no reproductive investment. Further analyses of the data showed that pregnant female pond bats are highly dependent on small prey and pupae, mainly nonbiting midges and mosquitoes (Diptera: Chironomidae and Culicidae). These insects can be found in large quantities in peatlands intersected with shallow waterways, the habitat type in which female pond bats were observed more often than males. Our results suggest that during pregnancy the spatial segregation of sexes coincides with sex-specific diets, which might reflect habitat selection based on energy requirements, in addition to lowered intraspecific competition.
Keywords: Chiroptera; Myotis dasycneme; environmental DNA (eDNA); high-throughput sequencing; intraspecific competition; metabarcoding.
© The Author(s) 2023. Published by Oxford University Press on behalf of the American Society of Mammalogists.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- Acharya L. 1995. Sex-biased predation on moths by insectivorous bats. Animal Behaviour 49:1461–1468.
-
- Aguirre L.F., Herrel A., Van Damme R., Matthysen E.. 2003. The implications of food hardness for diet in bats. Functional Ecology 17:201–212.
-
- Almenar D., Aihartza J., Goiti U., Salsamendi E., Garin I.. 2008. Diet and prey selection in the trawling long-fingered bat. Journal of Zoology 274:340–348.
-
- Arlettaz R. 1999. Habitat selection as a major resource partitioning mechanism between the two sympatric sibling bat species Myotis myotis and Myotis blythii. Journal of Animal Ecology 68:460–471.
LinkOut - more resources
Full Text Sources

