Changes in environmental and engineered conditions alter the plasma membrane lipidome of fractured shale bacteria
- PMID: 38059585
- PMCID: PMC10782966
- DOI: 10.1128/spectrum.02334-23
Changes in environmental and engineered conditions alter the plasma membrane lipidome of fractured shale bacteria
Abstract
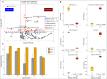
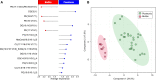
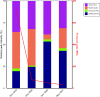
Microorganisms inadvertently introduced into the shale reservoir during fracturing face multiple stressors including brine-level salinities and starvation. However, some anaerobic halotolerant bacteria adapt and persist for long periods of time. They produce hydrogen sulfide, which sours the reservoir and corrodes engineering infrastructure. In addition, they form biofilms on rock matrices, which decrease shale permeability and clog fracture networks. These reduce well productivity and increase extraction costs. Under stress, microbes remodel their plasma membrane to optimize its roles in protection and mediating cellular processes such as signaling, transport, and energy metabolism. Hence, by observing changes in the membrane lipidome of model shale bacteria, Halanaerobium congolense WG10, and mixed consortia enriched from produced fluids under varying subsurface conditions and growth modes, we provide insight that advances our knowledge of the fractured shale biosystem. We also offer data-driven recommendations for improving biocontrol efficacy and the efficiency of energy recovery from unconventional formations.
Keywords: Halanaerobium; fractured shale; hydraulic retention time; intact polar lipids; lipidomics; membrane adaptation; salinity.
Conflict of interest statement
Fabrizio Colosimo is currently employed by New England Biolabs, Ipswich, MA, United States. Jishnu Adhikari is currently employed by Tetra Tech Inc., King of Prussia, PA, United States. The remaining authors declare no conflict of interest.
Figures





References
-
- Amundson KK, Borton MA, Daly RA, Hoyt DW, Wong A, Eder E, Moore J, Wunch K, Wrighton KC, Wilkins MJ. 2022. Correction to: microbial colonization and persistence in deep fractured shales is guided by metabolic exchanges and viral predation. Microbiome 10:30. doi:10.1186/s40168-022-01239-6 - DOI - PMC - PubMed
-
- Daly RA, Borton MA, Wilkins MJ, Hoyt DW, Kountz DJ, Wolfe RA, Welch SA, Marcus DN, Trexler RV, MacRae JD, Krzycki JA, Cole DR, Mouser PJ, Wrighton KC. 2016. Microbial metabolisms in a 2.5-km-deep ecosystem created by hydraulic fracturing in shales. Nat Microbiol 1:16146. doi:10.1038/nmicrobiol.2016.146 - DOI - PubMed
-
- Booker AE, Hoyt DW, Meulia T, Eder E, Nicora CD, Purvine SO, Daly RA, Moore JD, Wunch K, Pfiffner SM, Lipton MS, Mouser PJ, Wrighton KC, Wilkins MJ, Liu S-J. 2019. Deep-subsurface pressure stimulates metabolic plasticity in shale-colonizing Halanaerobium spp. Appl Environ Microbiol 85:e00018-19. doi:10.1128/AEM.00018-19 - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

