Bacteriophage infection and killing of intracellular Mycobacterium abscessus
- PMID: 38059609
- PMCID: PMC10790704
- DOI: 10.1128/mbio.02924-23
Bacteriophage infection and killing of intracellular Mycobacterium abscessus
Abstract
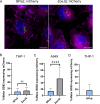
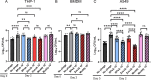
As we rapidly approach a post-antibiotic era, bacteriophage (phage) therapy may offer a solution for treating drug-resistant bacteria. Mycobacterium abscessus is an emerging, multidrug-resistant pathogen that causes disease in people with cystic fibrosis, chronic obstructive pulmonary disease, and other underlying lung diseases. M. abscessus can survive inside host cells, a niche that can limit access to antibiotics. As current treatment options for M. abscessus infections often fail, there is an urgent need for alternative therapies. Phage therapy is being used to treat M. abscessus infections as an option of last resort. However, little is known about the ability of phages to kill bacteria in the host environment and specifically in an intracellular environment. Here, we demonstrate the ability of phages to enter mammalian cells and to infect and kill intracellular M. abscessus. These findings support the use of phages to treat intracellular bacterial pathogens.
Keywords: Mycobacterium abscessus; bacteriophage therapy; intracellular bacteria; macrophages; nontuberculous mycobacteria.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Magiorakos A-P, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. 2012. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18:268–281. doi:10.1111/j.1469-0691.2011.03570.x - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
