Randomized adaptive selection trial of cryotherapy, compression therapy, and placebo to prevent taxane-induced peripheral neuropathy in patients with breast cancer
- PMID: 38060077
- PMCID: PMC10840989
- DOI: 10.1007/s10549-023-07172-y
Randomized adaptive selection trial of cryotherapy, compression therapy, and placebo to prevent taxane-induced peripheral neuropathy in patients with breast cancer
Abstract
Background: Chemotherapy-induced peripheral neuropathy (CIPN) is a common and debilitating adverse effect of taxane therapy. Small non-randomized studies in patients with early-stage breast cancer (ESBC) suggest both cryotherapy and compression therapy may prevent CIPN. It is unknown which is more effective.
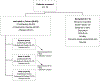
Methods: We conducted a randomized phase IIB adaptive sequential selection trial of cryotherapy vs. compression therapy vs. placebo ("loose" gloves/socks) during taxane chemotherapy. Participants were randomized in triplets. Garments were worn for 90-120 min, beginning 15 min prior and continuing for 15 min following the infusion. The primary goal was to select the best intervention based on a Levin-Robbins-Leu sequential selection procedure. The primary endpoint was a < 5-point decrease in the Functional Assessment of Cancer Therapy Neurotoxicity (FACT-NTX) at 12 weeks. An arm was eliminated if it had four or more fewer successes than the currently leading arm. Secondary endpoints included intervention adherence and patient-reported comfort/satisfaction.
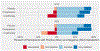
Results: Between April 2019 and April 2021, 63 patients were randomized (cryotherapy (20); compression (22); placebo (21)). Most patients (60.3%) were treated with docetaxel. The stopping criterion was met after the 17th triplet (n = 51) was evaluated; success at 12 weeks occurred in 11 (64.7%) on compression therapy, 7 (41.1%) on cryotherapy, and 7 (41.1%) on placebo. Adherence to the intervention was lowest with cryotherapy (35.0%) compared to compression (72.7%) and placebo (76.2%).
Conclusion: Compression therapy was the most effective intervention in this phase IIB selection trial to prevent CIPN and was well tolerated. Compression therapy for the prevention of CIPN should be evaluated in a phase III study.
Clinical trial registration: ClinicaTrials.gov Identifier: NCT03873272.
Keywords: Chemotherapy-induced peripheral neuropathy; Compression therapy; Cryotherapy; Taxane chemotherapy.
© 2023. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
Figures


References
-
- Windebank AJ, Grisold W: Chemotherapy-induced neuropathy. J Peripher Nerv Syst 2008, 13(1):27–46. - PubMed
-
- Chase DM, Huang H, Foss CD, Wenzel LB, Monk BJ, Burger RA: Neurotoxicity in ovarian cancer patients on Gynecologic Oncology Group (GOG) protocol 218: characteristics associated with toxicity and the effect of substitution with docetaxel: an NRG Oncology/Gynecologic Oncology Group study. Gynecol Oncol 2015, 136(2):323–327. - PMC - PubMed
-
- Miller DS, Filiaci VL, Mannel RS, Cohn DE, Matsumoto T, Tewari KS, DiSilvestro P, Pearl ML, Argenta PA, Powell MA et al.: Carboplatin and Paclitaxel for Advanced Endometrial Cancer: Final Overall Survival and Adverse Event Analysis of a Phase III Trial (NRG Oncology/GOG0209). J Clin Oncol 2020, 38(33):3841–3850. - PMC - PubMed
-
- Hershman DL, Weimer LH, Wang A, Kranwinkel G, Brafman L, Fuentes D, Awad D, Crew KD: Association between patient reported outcomes and quantitative sensory tests for measuring long-term neurotoxicity in breast cancer survivors treated with adjuvant paclitaxel chemotherapy. Breast Cancer Res Treat 2011, 125(3):767–774. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

