Efficacy and Safety of the Travoprost Intraocular Implant in Reducing Topical IOP-Lowering Medication Burden in Patients with Open-Angle Glaucoma or Ocular Hypertension
- PMID: 38060092
- PMCID: PMC10789685
- DOI: 10.1007/s40265-023-01973-7
Efficacy and Safety of the Travoprost Intraocular Implant in Reducing Topical IOP-Lowering Medication Burden in Patients with Open-Angle Glaucoma or Ocular Hypertension
Abstract
Purpose: A randomized, double-masked, multicenter, phase 2 trial to evaluate the long-term safety and efficacy of travoprost intraocular implant, an extended-release drug delivery system designed to provide uninterrupted sustained intraocular pressure (IOP)-lowering therapy, thereby reducing patient treatment burden and improving adherence with IOP-lowering medication.
Methods: Patients with open-angle glaucoma or ocular hypertension were administered a fast-eluting implant (FE implant, n = 51) and received twice-daily (BID) placebo eye drops, a slow-eluting (SE implant, n = 54) and received BID placebo eye drops, or underwent a sham surgical procedure and received BID timolol 0.5% (n = 49). IOP was measured at baseline, day 1-2, day 10, week 4, week 6, month 3, and every 3 months thereafter through 36 months. Efficacy was evaluated by mean change from 8:00 AM unmedicated baseline IOP through month 36, and the percentage of patients receiving the same or fewer topical IOP-lowering medications as at screening (pre-study). Safety was evaluated by adverse events and ophthalmic parameters.
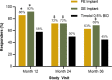
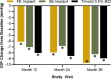
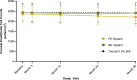
Results: Clinically and statistically relevant IOP-lowering treatment effects were observed through month 36 after a single administration of the travoprost implant compared with BID timolol with mean IOP reductions ranging from 7.6 to 8.8 mmHg for the FE implant group, from 7.3 to 8.0 mmHg for the SE implant group, and from 7.3 to 7.9 for the timolol group at the 8:00 AM timepoint (P < 0.0001 for all treatment groups at all visits). At months 12, 24, and 36, a greater percentage of FE and SE implant patients versus timolol patients were well controlled on the same or fewer topical IOP-lowering medications compared with screening with 63 and 69% for the FE and SE implants groups, respectively, versus 45% for the timolol group at month 36. The safety profile of the implant was favorable; there were no dislodgements, no explantations, no adverse events of conjunctival hyperemia or periorbital fat atrophy, no discontinuations due to study eye adverse events, nor any serious adverse events in the study eye. Comparable changes from baseline in corneal endothelial cell counts were observed in the three treatment groups over the 36 months.
Conclusion: The travoprost intraocular implant demonstrated robust IOP-lowering and substantially reduced topical IOP-lowering medication burden for up to 36 months following a single administration, while maintaining a favorable safety profile. The travoprost intraocular implant promises to be a meaningful addition to the interventional glaucoma armamentarium by addressing the key shortcomings of topical IOP-lowering medications, including low adherence and topical side effects while controlling IOP for up to 36 months.
Trial registry: ClinicalTrials.gov identifier NCT02754596 registered 28 April 2016.
© 2023. The Author(s).
Conflict of interest statement
John P. Berdahl has received consulting fees, paid advisory board member or received fees for attending a meeting for AbbVie, Aerpio, Alcon, Aldeyra, Aurea Medical, Aurion Biotech/CorneaGen, Balance Ophthalmics, Bausch and Lomb, Dakota Lions Eye Bank, Elios Vision Inc., Equinox, Expert Opinion, Glaukos, Gore, Imprimis, Interfeen, iRenix, Iacta Pharmaceuticals, IVERIC bio, Inc., JNJ, Kala, Kedalion, MELT Pharmaceuticals, MicroOptx, New World Medical, Ocular Surgical Data, Ocular Therapeutix, Omega Ophthalmic, Orasis, Oyster Point, RxSight, Santen, Sight Sciences, Surface Inc., Tarsus, Tear Clear, Vertex Ventures, ViaLase, Vittamed, Vance Thompson Vision, Versea Biologics, Visionary Ventures, Visus and Zeiss; has received lecture fees (honoraria), travel fees or reimbursements when speaking for AbbVie, Alcon and Glaukos; has equity ownership/stock options of Aurion Biotech/CorneaGen, Balance Ophthalmics, Equinox, Expert Opinion, Interfeen, Ocular Surgical Data, Omega Ophthalmic, Surface Inc, Vance Thompson Vision, Verana Health and Zeiss; owns stock of Glaukos; has patents and/or royalties with Imprimis. Steven R. Sarkisian Jr is a consultant/advisor for Alcon, Allergan, Bausch + Lomb, Beaver-Visitec International, Glaukos, Icare USA, Katena Products, MicroSurgical Technology, Ocular Science, Santen, and Sight Sciences; is on the speaker’s bureau for Alcon, Allergan, and Bausch + Lomb; has received grant support from Alcon, Allergan, Allysta Pharmaceuticals, Elios, Glaukos, iSTAR Medical, Ocular Science, Ocular Therapeutix, and Sight Sciences; and holds stock or stock options in Ocular Science and Sight Sciences. Robert E. Ang has been a speaker for, and received research support from Glaukos. Long V. Doan, L. Jay Katz, Angela C. Kothe, Dale W. Usner and Tomas Navratil are employees of Glaukos Corporation and may hold Glaukos stock/stock options.
Figures


References
-
- Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–713. doi: 10.1001/archopht.120.6.701. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

