Functional Contribution and Clinical Implication of Cancer-Associated Fibroblasts in Glioblastoma
- PMID: 38060213
- PMCID: PMC10922678
- DOI: 10.1158/1078-0432.CCR-23-0493
Functional Contribution and Clinical Implication of Cancer-Associated Fibroblasts in Glioblastoma
Abstract
Purpose: The abundance and biological contribution of cancer-associated fibroblasts (CAF) in glioblastoma (GBM) are poorly understood. Here, we aim to uncover its molecular signature, cellular roles, and potential tumorigenesis implications.
Experimental design: We first applied single-cell RNA sequencing (RNA-seq) and bioinformatics analysis to identify and characterize stromal cells with CAF transcriptomic features in human GBM tumors. Then, we performed functional enrichment analysis and in vitro assays to investigate their interactions with malignant GBM cells.
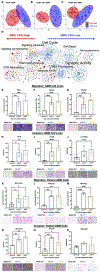
Results: We found that CAF abundance was low but significantly correlated with tumor grade, poor clinical outcome, and activation of extracellular matrix remodeling using three large cohorts containing bulk RNA-seq data and clinical information. Proteomic analysis of a GBM-derived CAF line and its secretome revealed fibronectin (FN1) as a critical candidate factor mediating CAF functions. This was validated using in vitro cellular models, which demonstrated that CAF-conditioned media and recombinant FN1 could facilitate the migration and invasion of GBM cells. In addition, we showed that CAFs were more abundant in the mesenchymal-like state (or subtype) than in other states of GBMs. Interestingly, cell lines resembling the proneural state responded to the CAF signaling better for the migratory and invasive phenotypes.
Conclusions: Overall, this study characterized the molecular features and functional impacts of CAFs in GBM, alluding to novel cell interactions mediated by CAFs in the GBM microenvironment.
©2023 American Association for Cancer Research.
Conflict of interest statement
Figures





References
-
- Wen PY, Weller M, Lee EQ, Alexander BM, Barnholtz-Sloan JS, Barthel FP, et al. Glioblastoma in adults: a Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro Oncol 2020;22(8):1073–113 doi 10.1093/neuonc/noaa106. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

