The Reporting and Methodological Quality of Systematic Reviews Underpinning Clinical Practice Guidelines Focused on the Management of Cutaneous Melanoma: Cross-Sectional Analysis
- PMID: 38060306
- PMCID: PMC10739238
- DOI: 10.2196/43821
The Reporting and Methodological Quality of Systematic Reviews Underpinning Clinical Practice Guidelines Focused on the Management of Cutaneous Melanoma: Cross-Sectional Analysis
Abstract
Background: Clinical practice guidelines (CPGs) inform evidence-based decision-making in the clinical setting; however, systematic reviews (SRs) that inform these CPGs may vary in terms of reporting and methodological quality, which affects confidence in summary effect estimates.
Objective: Our objective was to appraise the methodological and reporting quality of the SRs used in CPGs for cutaneous melanoma and evaluate differences in these outcomes between Cochrane and non-Cochrane reviews.
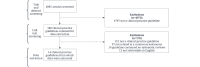
Methods: We conducted a cross-sectional analysis by searching PubMed for cutaneous melanoma guidelines published between January 1, 2015, and May 21, 2021. Next, we extracted SRs composing these guidelines and appraised their reporting and methodological rigor using the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) and AMSTAR (A Measurement Tool to Assess Systematic Reviews) checklists. Lastly, we compared these outcomes between Cochrane and non-Cochrane SRs. All screening and data extraction occurred in a masked, duplicate fashion.
Results: Of the SRs appraised, the mean completion rate was 66.5% (SD 12.29%) for the PRISMA checklist and 44.5% (SD 21.05%) for AMSTAR. The majority of SRs (19/50, 53%) were of critically low methodological quality, with no SRs being appraised as high quality. There was a statistically significant association (P<.001) between AMSTAR and PRISMA checklists. Cochrane SRs had higher PRISMA mean completion rates and higher methodological quality than non-Cochrane SRs.
Conclusions: SRs supporting CPGs focused on the management of cutaneous melanoma vary in reporting and methodological quality, with the majority of SRs being of low quality. Increasing adherence to PRISMA and AMSTAR checklists will likely increase the quality of SRs, thereby increasing the level of evidence supporting cutaneous melanoma CPGs.
Keywords: clinical; clinical practice guidelines; cutaneous melanoma; decision making; evidence; management; melanoma; practice guideline; review; systematic review.
©Mahnoor Khalid, Bethany Sutterfield, Kirstien Minley, Ryan Ottwell, McKenna Abercrombie, Christopher Heath, Trevor Torgerson, Micah Hartwell, Matt Vassar. Originally published in JMIR Dermatology (http://derma.jmir.org), 07.12.2023.
Conflict of interest statement
Conflicts of Interest: MV reports receipt of funding from the National Institute on Drug Abuse, the National Institute on Alcohol Abuse and Alcoholism, the US Office of Research Integrity, the Oklahoma Center for Advancement of Science and Technology, and internal grants from the Oklahoma State University Center for Health Sciences—all outside of the present work. MH reports funding from the National Institutes of Justice for unrelated work.
Figures
Similar articles
-
An Evaluation of Evidence Underpinning Management Recommendations in Tobacco Use Disorder Clinical Practice Guidelines.Nicotine Tob Res. 2022 Apr 28;24(6):847-854. doi: 10.1093/ntr/ntac012. Nicotine Tob Res. 2022. PMID: 35023556 Free PMC article.
-
Quality of reporting among systematic reviews underpinning the ESC/ACC guidelines on ventricular arrhythmias and sudden cardiac death.BMJ Evid Based Med. 2022 Dec;27(6):352-360. doi: 10.1136/bmjebm-2021-111859. Epub 2022 Mar 11. BMJ Evid Based Med. 2022. PMID: 35277437
-
Analysis of Systematic Reviews in Clinical Practice Guidelines for Head and Neck Cancer.Laryngoscope. 2022 Oct;132(10):1976-1983. doi: 10.1002/lary.30051. Epub 2022 Feb 14. Laryngoscope. 2022. PMID: 35156725
-
An Analysis of the Evidence Informing Clinical Practice Guidelines in the Management and Treatment of Breast Cancer.Clin Breast Cancer. 2022 Aug;22(6):588-600. doi: 10.1016/j.clbc.2022.04.009. Epub 2022 Apr 29. Clin Breast Cancer. 2022. PMID: 35676189
-
An Analysis of the Evidence Underpinning the American Urologic Association Clinical Practice Guidelines.Urology. 2022 Mar;161:42-49. doi: 10.1016/j.urology.2021.12.019. Epub 2022 Jan 2. Urology. 2022. PMID: 34986408 Review.
Cited by
-
Reliability and reproducibility of systematic reviews informing the 2020-2025 Dietary Guidelines for Americans: a pilot study.Am J Clin Nutr. 2025 Jan;121(1):111-124. doi: 10.1016/j.ajcnut.2024.10.013. Epub 2024 Dec 12. Am J Clin Nutr. 2025. PMID: 39755432 Free PMC article.
References
-
- Kredo T, Bernhardsson S, Machingaidze S, Young T, Louw Q, Ochodo E, Grimmer K. Guide to clinical practice guidelines: the current state of play. Int J Qual Health Care. 2016 Feb;28(1):122–8. doi: 10.1093/intqhc/mzv115. https://europepmc.org/abstract/MED/26796486 mzv115 - DOI - PMC - PubMed
-
- Murad MH. Clinical practice guidelines: a primer on development and dissemination. Mayo Clin Proc. 2017 Mar;92(3):423–433. doi: 10.1016/j.mayocp.2017.01.001. https://www.mayoclinicproceedings.org/article/S0025-6196(17)30025-3/full... S0025-6196(17)30025-3 - DOI - PubMed
-
- Wakkee M, Lugtenberg M, Spuls PI, de Jong EM, Thio HB, Westert GP, Nijsten T. Knowledge, attitudes and use of the guidelines for the treatment of moderate to severe plaque psoriasis among Dutch dermatologists. Br J Dermatol. 2008 Aug;159(2):426–32. doi: 10.1111/j.1365-2133.2008.08692.x.BJD8692 - DOI - PubMed
-
- Taba P, Rosenthal M, Habicht J, Tarien H, Mathiesen M, Hill S, Bero L. Barriers and facilitators to the implementation of clinical practice guidelines: a cross-sectional survey among physicians in Estonia. BMC Health Serv Res. 2012 Dec 13;12:455. doi: 10.1186/1472-6963-12-455. https://bmchealthservres.biomedcentral.com/articles/10.1186/1472-6963-12... 1472-6963-12-455 - DOI - DOI - PMC - PubMed
-
- Aran G, Hicks C, Demand A, Johnson AL, Beaman J, Bailey Y, Haught M, Lane A, Sinnett P, Vassar M. Treating schizophrenia: the quality of evidence behind treatment recommendations and how it can improve. BMJ Evid Based Med. 2020 Aug;25(4):138–142. doi: 10.1136/bmjebm-2019-111233.bmjebm-2019-111233 - DOI - PubMed
LinkOut - more resources
Full Text Sources